Abstract
Purpose
Colloid-facilitated migration of phosphorus (P) is a widely accepted phenomenon in surface and subsurface environment. Release and migration of colloidal P (Pcoll) in agricultural fields are closely related to P fertilization regimes. In this study, a site-specific experiment with rice/oilseed rape rotation was conducted to determine the export potential of Pcoll from the field and literatures reporting the impact of P fertilization regimes on release and migration of Pcoll in other agricultural fields were compared.
Materials and methods
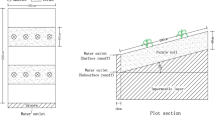
In this 2-year field experiment, four P fertilization regimes (no fertilizer control, inorganic P fertilizer of low and high rates, and swine manure treatment) with three replicates were conducted. Floodwater and runoff samples were collected in flooding season and the 100-cm-depth soil samples were collected after both crops’ harvest seasons. Colloidal particles were separated by microfiltration and ultracentrifugation processes and determined gravimetrically. The Pcoll value was calculated as the difference between the concentration of total P in non-ultracentrifuged and ultracentrifuged samples. The same method was applied for the colloidal mineral elements (Fe and Al) and organic carbon.
Results and discussion
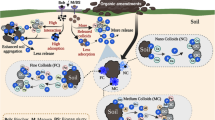
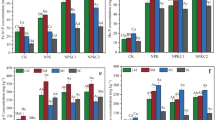
Total P concentration in paddy floodwater significantly increased after fertilization but decreased quickly in the following days, maintaining at 6.0 mg m−2. In soil extracts, concentration of Pcoll was low but stable, which ranged from 6 to 22 % of total P after oilseed rape season and from 7 to 18 % after rice season. In runoff samples, there were positive correlations between Pcoll, colloidal Fe (Fecoll), colloidal Al (Alcoll), and colloidal TOC (TOCcoll); the majority of P forms was molybdate reactive P. In both crops’ seasons, the amount of colloids increased with soil depth. Content of soil Pcoll was low and occupied 0.1–2 % of total P. The literature review showed that Pcoll in soil solution, runoff, and leachate ranged from 1.4 to 94 % of total P.
Conclusions
These results suggested that although the concentrations of Pcoll were not high, they widely distributed in paddy floodwater, runoff, and soil profile. Fertilization regimes and planting systems had a significant influence on the contents of Pcoll. Moreover, the Pcoll binding with Fe/Al minerals and organic carbon might be an alternative route of P loss in paddy field.




Similar content being viewed by others
References
Beck MA, Robarge WP, Buol SW (1999) Phosphorus retention and release of anions and organic carbon by two Andisols. Eur J Soil Sci 50:157–164
Buesseler KO, Kaplan DI, Dai M, Pike S (2009) Source-dependent and source-independent controls on plutonium oxidation state and colloid associations in groundwater. Environ Sci Technol 43:1322–1328
Chen D, Zheng A, Chen M (2010) Study of colloidal phosphorus variation in estuary with salinity. Acta Oceanol Sin 29:17–25
de Jonge LW, Kjaergaard C, Moldrup P (2004) Colloids and colloid-facilitated transport of contaminants in soils: an introduction. Vadose Zone J 3:321–325
Fuchs JW, Fox GA, Storm DE, Penn CJ, Brown GO (2009) Subsurface transport of phosphorus in riparian floodplains: influence of preferential flow paths. J Environ Qual 38:473–484
Gao Y, Zhu B, He N, Yu G, Wang T, Chen W, Tian J (2014) Phosphorus and carbon competitive sorption-desorption and associated non-point loss respond to natural rainfall events. J Hydrol 517:447–457
Garg AK, Aulakh MS (2010) Effect of long-term fertilizer management and crop rotations on accumulation and downward movement of phosphorus in semi-arid subtropical irrigated soils. Commun Soil Sci Plant Anal 41:848–864
Grant RF, Heaney DJ (1997) Inorganic phosphorus transformation and transport in soils: mathematical modeling in ecosys. Soil Sci Soc Am J 61:752–764
Guppy CN, Menzies NW, Blamey FPC, Moody PW (2005) Do decomposing organic matter residues reduce phosphorus sorption in highly weathered soils? Soil Sci Soc Am J 69:1405–1411
Hao X, Godlinski F, Chang C (2008) Distribution of phosphorus forms in soil following long-term continuous and discontinuous cattle manure applications. Soil Sci Soc Am J 72:90–97
Haygarth PM, Warwick MS, House WA (1997) Size distribution of colloidal molybdate reactive phosphorus in river waters and soil solution. Water Res 31:439–448
Heathwaite L, Haygarth P, Matthews R, Preedy N, Butler P (2005) Evaluating colloidal phosphorus delivery to surface waters from diffuse agricultural sources. J Environ Qual 34:287–298
Henderson R, Kabengi N, Mantripragada N, Cabrera M, Hassan S, Thompson A (2012) Anoxia-induced release of colloid- and nanoparticle-bound phosphorus in grassland soils. Environ Sci Technol 46:11727–11734
Hens M, Merckx R (2001) Functional characterization of colloidal phosphorus species in the soil solution of sandy soils. Environ Sci Technol 35:493–500
Hens M, Merckx R (2002) The role of colloidal particles in the speciation and analysis of “dissolved” phosphorus. Water Res 36:1483–1492
Ilg K, Siemens J, Kaupenjohann M (2005) Colloidal and dissolved phosphorus in sandy soils as affected by phosphorus saturation. J Environ Qual 34:926–935
Klitzke S, Lang F, Kaupenjohann M (2008) Increasing pH releases colloidal lead in a highly contaminated forest soil. Eur J Soil Sci 59:265–273
Kretzschmar R, Borkovec M, Grolimund D, Elimelech M (1999) Mobile subsurface colloids and their role in contaminant transport. Adv Agron 66:121–193
Li H, Huang G, Meng Q, Ma L, Yuan L, Wang F, Zhang W, Cui Z, Shen J, Chen X, Jiang R, Zhang F (2011) Integrated soil and plant phosphorus management for crop and environment in China. A review. Plant Soil 349:157–167
Liang X, Liu J, Chen Y, Li H, Ye Y, Nie Z, Su M, Xu Z (2010) Effect of pH on the release of soil colloidal phosphorus. J Soils Sediments 10:1548–1556
McGechan MB (2002) Effects of timing of slurry spreading on leaching of soluble and particulate inorganic phosphorus explored using the MACRO model. Biosyst Eng 83:237–252
Monbet P, McKelvie ID, Worsfold PJ (2010) Sedimentary pools of phosphorus in the eutrophic Tamar estuary (SW England). J Environ Monit 12:296–304
Naveed M, Moldrup P, Vogel HJ, Lamande M, Wildenschild D, Tuller M, de Jonge LW (2014) Impact of long-term fertilization practice on soil structure evolution. Geoderma 217:181–189
Regelink IC, Koopmans GF, van der Salm C, Weng L, van Riemsdijk WH (2013) Characterization of colloidal phosphorus species in drainage waters from a clay soil using asymmetric flow field-flow fractionation. J Environ Qual 42:464–473
Shand CA, Smith S, Edwards AC, Fraser AR (2000) Distribution of phosphorus in particulate, colloidal and molecular-sized fractions of soil solution. Water Res 34:1278–1284
Sharpley A (1995) Identifying sites vulnerable to phosphorus loss in agricultural runoff. J Environ Qual 24:947–951
Siemens J, Ilg K, Pagel H, Kaupenjohann M (2008) Is colloid-facilitated phosphorus leaching triggered by phosphorus accumulation in sandy soils? J Environ Qual 37:2100–2107
Stamm C, Fluhler H, Gachter R, Leuenberger J, Wunderli H (1998) Preferential transport of phosphorus in drained grassland soils. J Environ Qual 27:515–522
Tang Z, Wu L, Luo Y, Christie P (2009) Size fractionation and characterization of nanocolloidal particles in soils. Environ Geochem Health 31:1–10
Tsao TM, Chen YM, Wang MK (2011) Origin, separation and identification of environmental nanoparticles: a review. J Environ Monit 13:1156–1163
Turner BL, Kay MA, Westermann DT (2004) Colloidal phosphorus in surface runoff and water extracts from semiarid soils of the western united states. J Environ Qual 33:1464–1472
Ulen B, Snall S (2007) Forms and retention of phosphorus in an illite-clay soil profile with a history of fertilisation with pig manure and mineral fertilisers. Geoderma 137:455–465
Vendelboe AL, Moldrup P, Heckrath G, Jin Y, de Jonge LW (2011) Colloid and phosphorus leaching from undisturbed soil cores sampled along a natural clay gradient. Soil Sci 176:399–406
Wigginton NS, Haus KL, Hochella MF Jr (2007) Aquatic environmental nanoparticles. J Environ Monit 9:1306–1316
Zang L, Tian G, Liang X, Liu J, Peng G (2011) Effect of water-dispersible colloids in manure on the transport of dissolved and colloidal phosphorus through soil column. Afr J Agric Res 6:6369–6376
Zang L, Tian GM, Liang XQ, He MM, Bao QB, Yao JH (2013) Profile distributions of dissolved and colloidal phosphorus as affected by degree of phosphorus saturation in paddy soil. Pedosphere 23:128–136
Zhang Z, Zhang J, He R, Wang Z, Zhu Y (2007) Phosphorus interception in floodwater of paddy field during the rice-growing season in TaiHu lake basin. Environ Pollut 145:425–433
Zhang YQ, Wen MX, Li XP, Shi XJ (2014) Long-term fertilisation causes excess supply and loss of phosphorus in purple paddy soil. J Sci Food Agric 94:1175–1183
Zirkler D, Lang F, Kaupenjohann M (2012) “Lost in filtration”-the separation of soil colloids from larger particles. Colloids Surf A Physicochem Eng Asp 399:35–40
Acknowledgments
This research was supported by the National Natural Science Foundation of China (41522108, 41271314), Natural Science Foundation of Zhejiang Province (LR16B070001), and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Chengrong Chen
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 147 kb)
Rights and permissions
About this article
Cite this article
Liang, X., Jin, Y., Zhao, Y. et al. Release and migration of colloidal phosphorus from a typical agricultural field under long-term phosphorus fertilization in southeastern China. J Soils Sediments 16, 842–853 (2016). https://doi.org/10.1007/s11368-015-1290-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1290-4