Abstract
Purpose
The purpose of this study was to divide humic acids into several fractions by means of their dissolving in buffers with different pH values and to characterize obtained humic fractions with respect to their composition, structure, particle size, and charge. Relationships between determined characteristics of fractionated humic acids and pH values of used buffer solutions and the method of fractionation was investigated.
Materials and methods
Humic acids were fractionated by means of two different methods: the subsequent dissolution in buffers adjusted to different pH and the sequential dissolution in buffers with increasing pH values. Composition, structure, and properties of the obtained humic fractions were studied using elemental analysis, FT-IR spectroscopy, UV/VIS spectroscopy, and light scattering methods.
Results and discussion
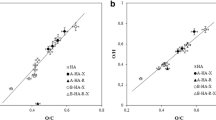
As expected, fractions obtained by subsequent dissolution were more heterogeneous than those prepared by sequential extraction. Fractions obtained at lower pH values contained higher amounts of aromatic and carboxylic groups, while those extracted at higher pH values were rich in aliphatic and/or peptide groups. Fractions extracted close to neutral pH region had some specific properties. Functional groups dissociated in a range of pH values depending on the chemical structure of the molecules. Weaker carboxylic groups could dissociate in less acidic solutions, and more aromatic fractions could be dissolved. Conformational changes and deaggregation process differed with the fractionation procedure and concentration of studied solutions. A bimodal distribution of particle sizes and higher values of polydispersity were obtained for some for less concentrated solutions of humic fractions.
Conclusions
Obtained humic fractions behave as anionic heterogeneous ligands with many carboxylic and phenolic groups of different strength, and present polyfunctional and polyelectrostatic effects due to different functional sites and net charged groups. Two main processes can affect their properties and behavior in aqueous solutions: dissociation of acidic functional groups and breaking of humic aggregates into smaller molecular associations or humic molecules. An important parameter affecting the spatial arrangement of obtained humic fractions is their concentration.




Similar content being viewed by others
References
Alvarez-Puebla RA, Garrido JJ (2005) Effect of pH on the aggregation of a gray humic acid in colloidal and solid states. Chemosphere 59:659–667
Angelico R, Ceglie A, Ji-Zheng H, Palumbo G, Colombo C (2014) Particle size, charge and colloidal stability of humic acids coprecipitated with ferrihydrite. Chemosphere 99:239–247
Atalay YB, Carbonaro RF, Di Toro DM (2009) Distribution of proton dissociation constants for model humic and fulvic acid molecules. Environ Sci Technol 43:3626–3631
Baalousha M, Motelico-Heino M, Le Coustumer P (2006) Conformation and size of humic substances: effects of major cation concentration and type, pH, salinity, and residence time. Colloid Surf A 272:48–55
Bakina LG, Orlova NE (2012) Special features of humus acids extraction from soils by sodium pyrophosphate solutions of different alkalinity. Euro Soil Sci 45:392–398
Barancikova G, Klucakova M, Madaras M, Makovnikova J, Pekar M (2003) Chemical structure of humic acids isolated from various soil types and lignite. Humic Subst Environ 3:3–8
Campitelli PA, Velasco MI, Ceppi SB (2006) Chemical and physicochemical characteristics of humic acids extracted from compost, soil and amended soil. Talanta 69:1234–1239
Chen Y, Senesi N, Schnitzer M (1977) Information provided on humic substances by E4/E6 ratios. Soil Sci Soc Am J 41:352–358
Chen J, Gu B, LeBoeuf EJ, Pan H, Dai S (2002) Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 48:59–68
Curtis MA, Witt AF, Schram SB, Rogers LB (1981) Humic acid fractionation using a nearly linear pH gradient. Anal Chem 53:1195–1199
Dai J, Ran W, Xing B, Gu M, Wang L (2006) Characterization of fulvic acid fractions obtained by sequential extractions with pH buffers, water, and ethanol from paddy soils. Geoderma 135:284–295
Du J, Sato N, Tochiyama O (2005) Potentiometric study on the proton binding of humic substances. J Nucl Radiochem Sci 6:25–29
Fuentes M, González-Gaitano G, García-Mina JM (2006) The usefulness of UV–visible and fluorescence spectroscopies to study the chemical nature of humic substances from soils and composts. Org Geochem 37:1949–1959
Fujitake N, Kusumoto A, Tsukamoto M, Kawahigashi M, Suzuki T, Otsuka H (1998) Properties of soil humic substances in fractions obtained by sequential extraction with pyrophosphate solutions at different pHs. I. Yield and particle size distribution. Soil Sci Plant Nutr 44:253–260
Fujitake N, Kusumoto A, Tsukamoto M, Noda Y, Suzuki T, Otsuka H (1999) Properties of soil humic substances in fractions obtained by sequential extraction with pyrophosphate solutions at different pHs. II. Elemental composition and UV–VIS spectra of humic acids. Soil Sci Plant Nutr 45:349–358
Fujitake N, Kusumoto A, Tsukamoto M, Yanagi Y, Suzuki T, Otsuka H (2003) Properties of soil humic substances in fractions obtained by sequential extraction with pyrophosphate solutions at different pHs. III. FT-IR and 1H NMR spectra of humic acids. Soil Sci Plant Nutr 49:347–353
Jovanovic UD, Markovic MM, Cupac SB, Tomic ZP (2013) Soil humic acid aggregation by dynamic light scattering and laser Doppler electrophoresis. J Plant Nutr Soil Sci 176:674–679
Kipton H, Powell HKJ, Town RM (1992) Solubility and fractionation of humic acid; effect of pH and ionic medium. Anal Chim Acta 267:47–54
Klucakova M (2010) Adsorption of nitrate on humic acids studied by flow-through coulometry. Environ Chem Lett 8:145–148
Klucakova M, Pekar M (2003) Study of structure and properties of humic and fulvic acids. IV. Study of interactions of Cu2+ ions with humic gels and final comparison. J Polym Mater 20:155–162
Klucakova M, Pekar M (2008) Behaviour of partially soluble humic acids in aqueous suspension. Colloid Surf A 318:106–110
Klucakova M, Pekar M (2009) Transport of copper(II) ions in humic gel—new results from diffusion couple. Colloid Surf A 349:96–101
Korshin GV, Li CW, Benjamin MM (1997) Monitoring the properties of natural organic matter through UV spectroscopy: a consistent theory. Water Res 3:1787–1795
Leenheer JA, Wershaw RL, Brown GK, Reddy MM (2003) Characterization and diagenesis of strong-acid carboxyl groups in humic substances. Appl Geochem 18:471–482
Marsalek R, Pospisil J, Taraba B (2011) The influence of temperature on the adsorption of CTAB on coals. Colloid Surf A 383:80–85
Peuravuori J, Zbankova P, Pihlaja K (2006) Aspects of structural features in lignite and lignite humic acids. Fuel Proc Technol 87:829–839
Peuravuori J, Simpson AJ, Lam B, Zbankova P, Pihlaja K (2007) Structural features of lignite humic acid in light of NMR and thermal degradation experiments. J Mol Struct 826:131–142
Piccolo A, Conte P, Cozzolino A (1999) Effects of mineral and monocarboxylic acids on the molecular association of dissolved humic substances. Eur J Soil Sci 50:687–694
Pinheiro JP, Mota AM, d’Oliveira JMR, Martinho JMG (1996) Dynamic properties of humic matter by dynamic light scattering and voltammetry. Anal Chim Acta 329:15–24
Schnitzer M, Kahn SU (1972) Humic substances in the environment. Marcel Dekker Inc, New York
Schnitzer M, Monreal CM (2011) Quo vadis soil organic matter research? A biological link to the chemistry of humification. Adv Agron 113:139–213
Senesi N, Plaza C, Brunetti G, Polo A (2007) A comparative survey of recent results on humic-like fractions in organic amendments and effects on native soil humic substances. Soil Biol Biochem 39:1244–1262
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions. Wiley, New York
Tan KH (2014) Humic matter in soil and the environment: principles and controversies. CRC Press, Boca Raton
Town RM, Kipton H, Powell HKJ (1992) Elimination of adsorption effects in gel permeation chromatography of humic substances. Anal Chim Acta 256:81–86
Yonebayashi K, Hattori T (1989) Chemical and biological studies on environmental humic acids. II. 1H NMR and IR spectra of humic acids. Soil Sci Plant Nutr 35:383–392
Yonebayashi K, Hattori T (1990) A new fractionation of soil humic acids by adsorption chromatography. Geoderma 47:327–336
You SJ, Thakali S, Allen HE (2006) Characteristics of soil organic matter (SOM) extracted using base with subsequent pH lowering and sequential pH extraction. Environ Int 32:101–105
Acknowledgments
This work was supported by Ministry of Education, Youth and Sports, Project LO1211
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Heike Knicker
Rights and permissions
About this article
Cite this article
Klučáková, M., Kalina, M. Composition, particle size, charge, and colloidal stability of pH-fractionated humic acids. J Soils Sediments 15, 1900–1908 (2015). https://doi.org/10.1007/s11368-015-1142-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1142-2