Abstract
Purpose
Carbon (C) flux is largely controlled by the highly bio-reactive labile C (LC) pool, while long-term C storage is determined by the recalcitrant C (RC) pool. Soil nitrogen (N) availability may considerably affect changes of these pools. The aim of this study was to investigate the effects of N treatments on soil LC and RC pools.
Materials and methods
A field experiment was conducted in a city lawn soil for 600 days with three N treatments, i.e., the control (0 kg N ha−1 year−1), low-N (100 kg N ha−1 year−1), and high-N (200 kg N ha−1 year−1) treatments. As the N source, NH4NO3 solution was added to soil surface monthly. Measurements of LC, RC, and other soil biochemical properties, including pH, soil respiration rates, microbial biomass, and enzymes activities, were taken during the experiment period.
Results and discussion
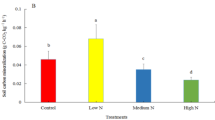
The low-N and high-N treatments increased 6.3 and 13% of the LC pool, respectively, which was caused by decreased microbial biomass and soil respiration rates under the N treatments. By contrary, the low-N and high-N treatments decreased 5.9 and 12% of the RC pool, respectively. The N addition treatments enhanced phenol oxidase activities. The enhanced oxidase activities decreased new RC input and the increased dissolved organic C stimulated RC pool decomposition. The LC and RC pools were highly influenced by the N treatments, whereas effect of the N treatments on soil organic C was not significant. The N addition treatments also caused soil acidification and reduced bacterial biomass proportion in the soil microbial composition.
Conclusions
The N addition increased the LC pool but decreased the RC pool in the soil. These changes should greatly impact soil long-term C storage.






Similar content being viewed by others
References
Allison SD, Vitousek PM (2004) Extracellular enzyme activities and carbon chemistry as drivers of tropical plant litter decomposition. Biotropica 36:285–296
Allison SD, Czimczik CI, Treseder KK (2008) Microbial activity and soil respiration under nitrogen additions in Alaskan boreal forest. Global Change Biol 14:1156–1168
Alvarez CR, Alvarez R, Grigera S, Lavado RS (1998) Associations between organic matter fractions and the active soil microbial biomass. Soil Biol Biochem 30(6):767–773
Bardgett RD, McAlister E (1999) The measurement of soil fungal: bacterial biomass ratios as an indicator of ecosystem self-regulation in temperate meadow grasslands. Biol Fert Soils 29:282–290
Bardgett RD, Lovell RD, Hobbs PJ, Jarvis SC (1999) Seasonal changes in soil microbial communities along a fertility gradient of temperate grasslands. Soil Biol Biochem 31:1021–1030
Bi J, Zhang NL, Liang Y, Yang HJ, Ma KP (2012) Interactive effects of water and nitrogen addition on soil microbial communities in a semiarid steppe. J Plant Ecol 5:320–329
Bossuyt H, Denef K, Six J, Frey SD, Merckx R, Paustian K (2001) Influence of microbial populations and residue quality on aggregate stability. Appl Soil Ecol 16:195–208
Bottomley PJ (1994) Light microscopic methods for studying soil microorganisms. In: Weaver RW (ed) Methods of soil analysis. Part 2. Microbiological and biochemical properties. Soil Science Society of America, Madison, pp 81–105
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Burton AJ, Pregitzer KS, Crawford JN, Zogg GP, Zak DR (2004) Simulated chronic NO3 − deposition reduces soil respiration in northern hardwood forests. Global Change Biol 10(7):1080–1091
Busse MD, Sanchez FG, Ratcliff AW, Butnor JR, Carter EA, Powers RE (2009) Soil carbon sequestration and changes in fungal and bacterial biomass following incorporation of forest residues. Soil Biol Biochem 41:220–227
Carrillo Y, Pendall E, Dijkstra FA, Morgan JA, Newcomb JM (2011) Response of soil organic matter pools to elevated CO2 and warming in a semi-arid grassland. Plant Soil 347:339–350
Cheng L, Leavitt SW, Kimball BA, Pinter JPJ, Ottman MJ, Matthias A, Wall GW, Brooks T, Williams DG, Thompson TL (2007) Dynamics of labile and recalcitrant soil carbon pools in a sorghum free-air CO2 enrichment (FACE) agro ecosystem. Soil Biol Biochem 39:2250–2263
Collins HP, Elliott ET, Paustian K, Bundy LG, Dick WA, Huggins DR, Smucker AJM, Paul EA (2000) Soil carbon pools and fluxes in long-term corn belt agroecosystems. Soil Biol Biochem 32:157–168
Currey PM, Johnson D, Sheppard LJ, Leith ID, Toberman H, Wal RV, Dawson LA, Artz RRE (2010) Turnover of labile and recalcitrant soil carbon differ in response to nitrate and ammonium deposition in an ombrotrophic peat land. Global Change Biol 16:2307–2321
DeForest JL, Zak DR, Pregitzer KS, Burton AJ (2004) Atmospheric nitrate deposition, microbial community composition, and enzyme activity in northern hardwood forests. Soil Sci Soc Am J 68:132–138
Emmett BA (1999) The impact of nitrogen on forest soils and feedbacks on tree growth. Water Air Soil Poll 116(1–2):65–74
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Fontaine S, Barot S, Barre P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280
Galantini J, Rosell R (2006) Long-term fertilization effects on soil organic matter quality and dynamics under different production systems in semi-arid Pampean soils. Soil Till Res 87:72–79
George E, Kircher S, Schwarz P, Tesar A, Seith B (1999) Effect of varied soil nitrogen supply on growth and nutrient uptake of young Norway spruce plants grown in a shaded environment. J Plant Nutr Soil Sci 162(3):301–307
Giesler R, Hogberg MN, Strobel BW, Richter A, Nordgren A, Hogberg P (2007) Production of dissolved organic carbon and low-molecular weight organic acids in soil solution driven by recent tree photosynthate. Biogeochemistry 84(1):1–12
Gong W, Yan X, Wang J (2012) The effect of chemical fertilizer on soil organic carbon renewal and CO2 emission—a pot experiment with maize. Plant Soil 353:85–94
Groffman PM, Williams CO, Pouyat RV, Band LE, Yesilonis ID (2009) Nitrate leaching and nitrous oxide flux in urban forests and grasslands. J Environ Qual 38:1848–1860
Helmisaari HS, Hallvacken L (1999) Fine-root biomass and necromass in limed and fertilized Norway spruce (Picea abies (L.) Karst.) stands. For Ecol Manag 119:99–110
Helmisaari HS, Saarsalmi A, Kukkola M (2009) Effects of wood ash and nitrogen fertilization on fine root biomass and soil and foliage nutrients in a Norway spruce stand in Finland. Plant Soil 314(1–2):121–132
Hobbie SE, Nadelhoffer KJ, Högberg P (2002) A synthesis: the role of nutrients as constraints on carbon balances in boreal and arctic regions. Plant Soil 242:163–170
Högberg MN, Högberg P (2002) Extramatrical ectomycorrhizal mycelium contributes one-third of microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil. New Phytol 154(3):791–795
Hu S, Coleman DC, Carroll CR, Hendrix PF, Beare MH (1997) Labile soil carbon pools in subtropical forest and agricultural ecosystems as influenced by management practices and vegetation types. Agr Ecosyst Environ 65:69–78
Huang Z, Clinton PW, Baisden WT, Davis MR (2011) Long-term nitrogen additions increased surface soil carbon concentration in a forest plantation despite elevated decomposition. Soil Biol Biochem 43:302–307
Ingham ER, Klein DA (1984) Soil fungi: relationships between hyphal activity and staining with fluorescein diacetate. Soil Biol Biochem 16:273–278
Iyyemperumal K, Shi W (2008) Soil enzyme activities in two forage systems following application of different rates of swine lagoon effluent or ammonium nitrate. Appl Soil Ecol 38:128–136
Jim CY, Chen WY (2006) Perception and attitude of residents toward urban green spaces in Guangzhou (China). Environ Manage 38:338–349
Johnsona D, Leakea JR, Leea JA, Campbell CD (1998) Changes in soil microbial biomass and microbial activities in response to 7 years simulated pollutant nitrogen deposition on a heathland and two grasslands. Environ Pollut 103:239–250
Keeler BL, Hobbie SE, Kellogg LE (2009) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15
Kepner RL, Pratt JT (1994) Use of fluorochromes for direct enumeration of total bacteria in environmental samples: past and present. Microbiol Mol Biol R 58:603–615
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371
Lal R, Kimble JM, Follett RF (1997) Pedospheric processes and the carbon cycle. In: Lal R, Kimble JM, Follett RF, Stewart BA (eds) Soil processes and the carbon cycle. CRC, New York, pp 1–8
Leavitt SW, Follett RF, Paul EA (1996) Estimation of slow- and fast-cycling soil organic carbon pools from 6 N HCl hydrolysis. Radiocarbon 38:231–239
Lin Z, Zhang R, Tang J, Zhang J (2010) Effects of high soil water content and temperature on soil respiration. Soil Sci 176:150–155
Lu X, Mo J, Gundersern P, Zhu W, Zhou G, Li D, Zhang X (2009) Effect of simulated N deposition on soil exchangeable cations in three forest types of subtropical China. Pedosphere 19(2):189–198
Magill AH, Aber JD, Hendricks JJ, Bowden RD, Melillo JM, Steudler PA (1997) Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol Appl 7(2):402–447
Micks P, Aber JD, Boone RD, Davidson EA (2004) Short-term soil respiration and nitrogen immobilization response to nitrogen applications in control and nitrogen-enriched temperate forests. Forest Ecol Manag 196(1):57–70
Paul EA, Morris SJ, Conant RT, Plante AF (2006) Does the acid hydrolysis-incubation method measure meaningful soil organic carbon pools? Soil Sci Soc Am J 70:1023–1035
Personeni E, Luscher A, Loiseau P (2005) Rhizosphere activity, grass species and N availability effects on the soil C and N cycles. Soil Biol Biochem 37(5):819–827
Pietri JCA, Brookes PC (2009) Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol Biochem 41:1396–1405
Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microb 75:1589–1596
Rovira P, Vallejo VR (2002) Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: an acid hydrolysis approach. Geoderma 107:109–141
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term N deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Sinsabaugh RL, Klug MJ, Collins HP, Yeager PE, Petersen SO (1999) Characterizing soil microbial communities. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 318–348
Sjöberg G, Knicker H, Nilsson SI, Berggren D (2004) Impact of long-term N fertilization on the structural composition of spruce litter and mor humus. Soil Biol Biochem 36:609–618
Snajdr J, Valaskova V, Merhautova V, Herinkova J, Cajthaml T, Baldrian P (2008) Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol Biochem 40:2068–2075
Söderström BE (1977) Vital staining of fungi in pure cultures and in soil with fluorescein diacetate. Soil Biol Biochem 9:59–63
Trumbore SE, Bonani G, Wolfli W (1990) The rates of carbon cycling in several soils from AMS 14C measurements of fractionated soil organic matter. In: Bouwman AF (ed) Soils and the greenhouse effect. Wiley, New York, pp 405–414
Waldrop MP, Zak DR, Sinsabaugh RL (2004) Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36:1443–1451
Zhang N, Wan S, Li L, Bi J, Zhao M, Ma K (2008) Impacts of urea N addition on soil microbial community in a semi-arid temperate steppe in northern China. Plant Soil 311:19–28
Zheng X, Fu C, Xu X, Xiao D, Huang Y, Chen G, Han S, Hu F (2002) The Asian nitrogen cycle case study. Ambio 31:79–87
Acknowledgments
This work was partly supported by grants from the Chinese National Natural Science Foundation (Nos. 51039007 and 51179212) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Jiang, X., Cao, L. & Zhang, R. Changes of labile and recalcitrant carbon pools under nitrogen addition in a city lawn soil. J Soils Sediments 14, 515–524 (2014). https://doi.org/10.1007/s11368-013-0822-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0822-z