Abstract
Purpose
Sorption and precipitation of phosphate are important processes in controlling fate of phosphorus (P) in P-fertilized soils, especially those affected by magnesium (Mg) ions.
Materials and methods
The interaction between Mg(II) (0.42 and 8.33 mM) ions and phosphate (0.32 and 6.45 mM) at the calcite–water interface were investigated with various pH values from 6.0 to 12.0, using a combination of sorption envelopes, Fourier transform infrared spectroscopy, and X-ray diffraction.
Results and discussion
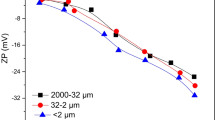
Amorphous calcium phosphate, dibasic calcium phosphate dihydrate, and hydroxyapatite are formed at high phosphate concentration (6.45 mM) and high pH (>8.0). The presence of low Mg(II) ion level (0.42 mM) had little effect on phosphate sorption. When Mg(II) ions increased to 8.33 mM, phosphate retention was inhibited in the weak acid condition since incorporation of Mg(II) ions kinetically hinders precipitation resulting in greater solubility of calcium phosphate while high pH favors Mg adsorption to provide more =Mg sites and OH functional groups on the surface of calcite, which enhanced the formation of Mg–P phases. The likely mechanism is attributed to the different surface terminations of calcite sorbed by phosphate at pH < 8.0 and pH > 8.0 in the presence of Mg(II) ions.
Conclusions
Our experimental results suggested that soil pH, initial concentration of phosphate, and the presence of Mg(II) ions and calcite play an important role to affect the fate of phosphate in P-fertilized soils.






Similar content being viewed by others
References
Alvarez R, Evans LA, Milham PJ, Wilson MA (2004) Effects of humic material on the precipitation of calcium phosphate. Geoderma 118:245–260
Arai Y, Sparks DL (2001) ATR–FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite–water interface. J Colloid Interface Sci 241:317–326
Arvidson RS, Collier M, Davis KJ, Vinson MD, Amonette JE, Luttge A (2006) Magnesium inhibition of calcite dissolution kinetics. Geochim Cosmochim Acta 70:583–594
Beauchemin S, Hesterberg D, Chou J, Beauchemin M, Simard RR, Sayers DE (2003) Speciation of phosphorus in phosphorus-enriched agricultural soils using X-ray absorption near-edge structure spectroscopy and chemical fractionation. J Environ Qual 32:1809–1819
Ben-Nissan B, Chai C, Evans L (1995) Crystallographic and spectroscopic characterization and morphology of biogenic and synthetic apatites. In: Wise DL et al (eds) Encyclopedic handbook of biomaterials and bioengineering: part B. Applications, Marcel Dekker, New York, USA, pp 191–221
Bertoluzza A, Bottura G, Taddei P, Tinti A (1996) Vibrational spectra of controlled-structure hydroxyapatite coatings obtained by the polymeric route. J Raman Spectrosc 27:759–764
Berzina-Cimdina L, Borodajenko N (2012) Research of calcium phosphates using Fourier transform infrared spectroscopy. In: Theophile T (ed) Infrared spectroscopy–materials science. Engineering and Technology. InTech China, Shanghai, pp 123–148
Cao X, Harris W (2008) Carbonate and magnesium interactive effect on calcium phosphate precipitation. Environ Sci Technol 42:436–442
Cao X, Harris WG, Josan MS, Nair VD (2007) Inhibition of calcium phosphate precipitation under environmentally-relevant conditions. Sci Total Environ 383:205–215
Elzinga EJ, Sparks DL (2007) Phosphate adsorption onto hematite: an in situ ATR-FTIR investigation of the effects of pH and loading level on the mode of phosphate surface complexation. J Colloid Interface Sci 308:53–70
Fenter P, Geissbühler P, DiMasi E, Srajer G, Sorensen LB, Sturchio NC (2000) Surface speciation of calcite observed in situ by X-ray scattering. Geochim Cosmochim Acta 64(7):1221–1228
Ferguson JF, McCarty PL (1971) Effects of carbonate and magnesium on calcium phosphate precipitation. Environ Sci Technol 5:534–540
Freeman JS, Rowell DLJ (1981) The adsorption and precipitation of phosphate onto calcite. Soil Sci 32:75–84
Gustafsson JP (2011) Visual MINTEQ version 3.0. http://www2.lwr.kth.se/English/OurSoftware/vminteq. Stockholm, Sweden. August 2007
Halmann M, Steinberg M (1999) Greenhouse gas carbon dioxide mitigation. Lewis Publications, London, UK, Science and Technology
Harris WG, Wang HD, Reddy KR (1994) Dairy manure influence on soil and sediment composition: implication for phosphorus pretension. J Environ Qual 23:1071–1081
He ZL, Baligar VC, Ritchey KD, Martens DC (1998) Determination of soluble phosphorus in the presence of organic ligands or fluoride. Soil Sci Soc Am J 62:1538
Hinedi ZR, Goldberg S, Chang AC, Yesinowski JP (1992) A 31P and 1H MAS NMR study of phosphate sorption onto calcium carbonate. J Colloid Interface Sci 152:141–160
House WA, Denison FH (2000) Factors influencing the measurement of equilibrium phosphate concentrations in river sediments. Water Res 34:1187–1200
House WA, Donaldson L (1986) Adsorption and coprecipitation of phosphate on calcite. J Colloid Interface Sci 112:69–80
House WA, Casey H, Donaldson L, Smith S (1986) Factors affecting the coprecipitation of inorganic phosphate with calcite in hardwaters–I, laboratory studies. Water Res 20:917–922
Hunger S, Cho H, Sims JT, Sparks DL (2004) Direct speciation of phosphorus in alum-amended poultry litter: solid-state 31P NMR investigation. Environ Sci Technol 38:674–681
Jiang GM (2007) Act on the remediation of contaminations in corp soil (in Chinese). Environ Econ 42:64–65
Johnson BB, Ivanov AV, Antzutkin ON, Forsling W (2002) 31P Nuclear magnetic resonance study of the adsorption of phosphate and phenyl phosphates on γ-Al2O3. Langmuir 18:1104–1111
Karaca S, Gürses A, Ejder M, Açikyildiz M (2004) Kinetic modeling of liquid-phase adsorption of phosphate on dolomite. J Colloid Interface Sci 277:257–263
Karageorgiou K, Paschalis M, Anastassakis GN (2007) Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J Hazard Mater 139:447–452
Kibalczyc W, Christoffersen J, Christoffersen MR, Zielenkiewicz A, Zielenkiewicz W (1990) The effect of magnesium-ions on the precipitation of calcium phosphates. J Cryst Growth 106:355–366
Lee YB, Kim PJ (2007) Reduction of phosphate adsorption by ion competition with silicate in soil. Korean J Environ Agric 26:286–293
Lin Y, Singer PC (2006) Inhibition of calcite precipitation by orthophosphate: speciation and thermodynamic considerations. Geochim Cosmochim Acta 70:2530–2539
Lyklema J (1989) Discrimination between physical and chemical adsorption of ions on oxides. Colloids Surf 37:197–204
Merino E, Canals À (2011) Self-accelerating dolomite-for-calcite replacement: self-organized dynamics of burial dolomitization and associated mineralization. Am J Sci 311:573–607
Millero F, Huang F, Zhu XR, Liu XW, Zhang JZ (2001) Adsorption and desorption of phosphate on calcite and aragonite in seawater. Aquat Geochem 7:33–56
Pokrovsky OS, Mielczarski JA, Barres O, Schott J (2000) Surface speciation models of calcite and dolomite/aqueous interfaces and their spectroscopic evaluation. Langmuir 16:2677–2688
Ruiz-Agudo E, Kowacz M, Putnis CV, Putnis A (2010) The role of background electrolytes on the kinetics and mechanism of calcite dissolution. Geochim Cosmochim Acta 74:1256–1267
Ruiz-Agudo E, Putnis CV, Rodriguez-Navarro C, Putnis A (2011) Effect of pH calcite growth at constant α Ca2+/ α CO3 2- ratio and supersaturation. Geochim Cosmochim Acta 75:284–296
Salimi MH, Heughebaert JC, Nancollas GH (1985) Crystal growth of calcium phosphates in the presence of magnesium ions. Langmuir 1:119–122
Sato S, Solomon D, Hyland C, Ketterings QM, Lehmann J (2005) Phosphorus speciation in manure and manure-amended soils using XANES spectroscopy. Environ Sci Technol 39:7485–7491
Sawada K (1998) Mechanisms of crystal growth of ionic crystals in solution. Formation, transformation, and growth inhibition of calcium carbonates. In: Ohtaki H (ed) Crystallization processes. John Wiley & Sons Ltd, Chichester, England, pp 39–68
Shariatmadari H, Mermut AR (1999) Magnesium and silicon-induced phosphate desorption in smectite, playgorskite, and sepiolite-calcite systems. Soil Sci Soc Am J 63:1167–1173
Shin EW, Han JS, Jang M, Min SH, Park JK, Rowell RM (2004) Phosphate adsorption on aluminum-impregnated mesoporous silicates: surface structure and behavior of adsorbents. Environ Sci Technol 38:912–917
Sims JT, Edwards AC, Schoumans OF, Simard RR (2000) Integrating soil phosphorus testing into environmentally best agricultural management practices. J Environ Qual 29:60–71
Snoeyink VL, Jenkins D (1980) Precipitation and dissolution, In: Water chemistry, John Wiley & Sons, New York, pp 243–315
SO HU, Postma D, Jakonsen R, Larsen F (2011) Sorption of phosphate onto calcite results from batch experiments and surface complexation modeling. Geochim Cosmochim Acta 75:2911–2923
Somasundaran P, Agar GE (1967) The zero point of charge of calcite. J Colloid Interface Sci 24:433–440
Stumm W (1992) Chemsitry of solid–water interface. Wiley, New York, p 428
Stumm W, Leckie JO (1970) Phosphate exchange with sediments; its role in the productivity of surface waters. Adv Water Pollut Res 26:1–16
Stumm W, Morgan JJ (1981) Aquatic chemistry—an introduction emphasizing chemical equilibria in natural waters DOI:10.1021/ed048pA779.1 (2ed.). John Wiley and Sons, New York
Suchanek WJ, Byrappa K, Shuk P, Riman RE, Janas VF, Tenhuisen KS (2004) Mechanochemical–hydrothermal synthesis of calcium phosphate powders with coupled magnesium and carbonate substitution. J Solid State Chem 17:793–799
Suzuki T, Inomata S, Sawada K (1986) Adsorption of phosphate on calcite. J Chem Soc Faraday Trans 82:1733–1743
Valsami-Jones E (2001) Mineralogical controls on phosphorus recovery from wastewaters. Mineral Mag 65:611–620
Van der Weijden RD, Comans RNJ (1997) Sorption and sorption reversibility of cadmium on calcite in the presence of phosphate and sulfate. Mar Chem 57:119–132
Vinson MD, Arvidson RS, Luttge A (2007) Kinetic inhibition of calcite (104) dissolution by aqueous manganese(II). J Crys Growth 307:116–125
Wang L, Ruiz-Agudo E, Putnis CV, Menneken M, Putnis A (2012) Kinetics of calcium phosphate nucleation and growth on calcite: implications for predicting the fate of dissolved phosphate species in alkaline soils. Environ Sci Technol 46:834–842
Wesolowski DJ, Machesky ML, Ridley MK, Palmer DA, Zhang Z, Fenter P, Předota M, Cummings PT (2008) Ion adsorption on metal oxide surfaces to hydrothermal conditions. ECS Trans 11:167–175
Xu N, Yin HW, Chen ZG, Chen M, Liu SQ (2013) Mechanisms of phosphate adsorption on synthesis calcite. Mater Sci Forum 743–744:597–602
Yadav BR, Paliwal KV, Nimgade NM (1984) Effect of magnesium-rich waters on phosphate adsorption by calcite. Soil Sci 138:153–157
Yagi S, Fukushi K (2011) Phosphate sorption on monohydrocalcite. J Miner Pet Sci 1066:109–113
Zachara JM, Cowan CE, Resch CT (1991) Sorption of divalent metals on calcite. Geochim Cosmochim Acta 55:1549–1562
Zhang T, Chen Y, Zhao L (2010) Countermeasures on Tianjin Ninghe rural NPS pollution control. Haihe river basin research and planning approach—proceedings of 2009 international symposium of Haihe basin integrated water and environment management. 5:110–115
Zhao H, Robert S (2001) Competitive adsorption of phosphate and arsenate on goethite. Environ Sci Technol 35:4753–4757
Zhou JB, Yang SL, Yu JG (2011) Facile fabrication of mesoporous MgO microspheres and their enhanced adsorption performance for phosphate from aqueous solutions. Colloid Surf A 379:102–108
Acknowledgments
This work has been supported by the National Natural Science Foundation of China (grant no. 21107077, 21377090, and 21071107), Natural Science Foundation of Jiangsu Province ((grant no. BK20131152), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The authors thank Dr. Taoyun Wang, Dr. Hongying Cheng, Shuling Dong, and li Li for their support with the analytical needs of the project respectively and Peter Malczyk for his collaboration in the production of the manuscript. The authors would also like to acknowledge the insightful comments of anonymous reviews in the production of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Xu, N., Yin, H., Chen, Z. et al. Mechanisms of phosphate retention by calcite: effects of magnesium and pH. J Soils Sediments 14, 495–503 (2014). https://doi.org/10.1007/s11368-013-0807-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0807-y