Abstract
Purpose
The binary competitive effect could obviously influence the fate and transport behavior of oxytetracycline (OTC) and cadmium (Cd2+) in cinnamon soil. However, two pollutants loading into soil usually are different, perhaps because of the three reasons including occurrence of OTC before Cd2+, simultaneous occurrence of OTC and Cd2+, or occurrence of Cd2+ before OTC. The purpose of the study was to predict the competitive adsorption and desorption of OTC and Cd2+ as a function of above input loadings on cinnamon soil.
Materials and methods
Adsorption and desorption were determined using the batch equilibrium method in a single or binary system. The Freundlich equation was applied to describe the adsorption/desorption data of OTC and Cd2+ in order to obtain adsorption/desorption isotherms for each tested compound and the respective adsorption/desorption coefficients.
Results and discussion
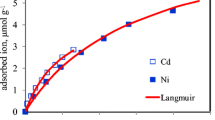
The results indicated that cinnamon soil could strongly adsorb OTC with the adsorption affinity (K f value) of more than 718 and Cd2+ with K f value of more than 536 in the competitive and non-competitive system, and all adsorption and desorption isotherms of OTC and Cd2+ on cinnamon soil were well fitted by the Freundlich equation with r value of more than 0.99 (p < 0.01). The coexistence of OTC and Cd2+ on cinnamon soil promoted significantly Cd2+ adsorption when Cd2+ firstly or simultaneously occurred on soil, but their coexistence did not affect adsorption of OTC when OTC firstly or simultaneously occurred on soil. Among the three input loadings, the pollutant with later occurring mode had lower K f and hysteresis coefficient (HI) than the other two input loadings. According to the adsorption intensity parameter (1/n), the presence of Cd2+ or OTC with different input loadings could decrease the adsorption intensity of OTC or Cd2+ when compared with single occurrence of OTC or Cd2+.
Conclusions
The binary competitive effect influenced the adsorption/desorption of OTC and Cd2+ differently. The presence of OTC had a stronger influence on the adsorption/desorption of Cd2+ than the presence of Cd2+ on the adsorption of OTC. The later occurring pollutant on soil had stronger ecological risk than the former occurring pollutant in the binary competitive system. The physical adsorption in the single or binary system could be identified as the dominant mechanisms of OTC and Cd2+ adsorption.


Similar content being viewed by others
References
Bao YY, Zhou QX, Wan Y, Yu Q, Xie XJ (2010) Effects of soil/solution ratios and cation types on adsorption and desorption of tetracycline in soils. Soil Sci Soc Am J 74:1553–1561
Barriuso E, Laird A, Koskinen WC, Dowdy RH (1994) Atrazine desorption from smectites. Soil Sci Soc Am J 58:1632–1638
Blackwell PA, Kay P, Boxall ABA (2007) The dissipation and transport of veterinary antibiotics in a sandy loam soil. Chemosphere 67:292–299
Calvet R (1989) Adsorption of organic chemicals in soil. Environ Health Perspect 83:145–177
Carter MC, Kilduff JE, Weber WJ (1995) Site energy distribution analysis of preloaded adsorbents. Environ Sci Technol 29:1773–1780
Chiou CT, Kile DE (1998) Deviations from sorption linearity on soils of polar and nonpolar organic compounds at low relative concentrations. Environ Sci Technol 32:338–343
Green RE, Yamane VK (1970) Precision in pesticide adsorption measurement. Soil Sci Soc Am J 34:353–355
Gu C, Karthikeyan KG (2005) Interaction of tetracycline with aluminum and iron hydrous oxides. Environ Sci Technol 39:2660–2667
Halling-Sørensen B (2000) Algal toxicity of antibacterial agents used in intensive farming. Chemosphere 40:731–739
Jjemba PK (2002) The potential impact of veterinary and human therapeutic agents in manure and biosolids on plants grown on arable land: a review. Agr Ecosyst Environ 1918:1–12
Kim S, Jensen JN, Aga DS, Weber AS (2007) Tetracycline as a selector for resistant bacteria in activated sludge. Chemosphere 66:1643–1651
Kulshrestha P, Giese RF, Aga DS (2004) Investigating the molecular interactions of oxytetracycline in clay and organic matter: insights on factors affecting its mobility in soil. Environ Sci Technol 38:4097–4105
Li YX, Xiong X, Lin CY, Zhang FS, Li W, Han W (2010) Cadmium in animal production and its potential hazard on Beijing and Fuxin farmlands. J Hazard Mater 177:475–480
OECD (2000) Guidelines for the testing of chemicals. In: Adsorption/desorption using a batch equilibrium method, OECD Test Guideline, vol. 106. OECD, Paris
Pateiro-Moure M, Arias-Estévez M, Simal-Gándara J (2010) Competitive and non-competitive adsorption/desorption of paraquat, diquat and difenzoquat in vineyard-devoted soils. J Hazard Mater 178:194–201
Pignatello JJ (1991) Competitive effects in the sorption of nonpolar organic compound by soils. In: Baker RA (ed) Organic substances and sediments in water. Lewis Publishers, Chelsea, pp 291–307
Sanderson H, Ingerslev F, Brain RA, Halling-Sørensen B, Bestari JK, Wilson CJ, Johnson DJ, Solomon KR (2005) Dissipation of oxytetracycline, chlortetracycline, tetracycline and doxycycline using HPLC-UV and LC/MS/MS under aquatic semi-field microcosm conditions. Chemosphere 60:619–629
Sarmah AK, Meyer MT, Boxall ABA (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759
Sassman SA, Lee LS (2005) Sorption of three tetracyclines by several soils: assessing the role of pH and cation exchange. Environ Sci Technol 39:7452–7459
Schmitt MO, Schneider S (2000) Spectroscopic investigation of complexation between various tetracyclines and Mg2+ or Ca2+. Phys Chem Commun 9:1–14
Singh N (2002) Sorption behavior of triazole fungicides in Indian soils and its correlation with soil properties. J Agric Food Chem 38:138–141
Tongaree S, Flanagan DR, Poust RI (1999) The interaction between oxytetracycline and divalent metal ions in aqueous and mixed solvent systems. Pharm Dev Technol 4:581–591
Wan Y, Bao YY, Zhou QX (2010) Simultaneous adsorption and desorption of cadmium and tetracycline on cinnamon soil. Chemosphere 80:807–812
Wang YJ, Jia DA, Sun RJ, Zhu HW, Zhou DM (2008) Adsorption and cosorption of tetracycline and copper(II) on montmorillonite as affected by solution pH. Environ Sci Technol 42:3254–3259
Wu ST (2005) The latest development about the remedy of Cd-contaminated soil. Guangdong Chem Ind 4:40–42
Xing BS, Pignatello JJ, Gigliotti B (1996) Competitive sorption between atrazine and other organic compounds in soils and model sorbents. Environ Sci Technol 30:2432–2440
Zhang ZY, Sun K, Gao B, Zhang GX, Liu XT, Ye Z (2011) Adsorption of tetracycline on soil and sediment: effects of pH and the presence of Cu(II). J Hazard Mater 190:856–862
Zhao YP, Geng JJ, Wang XR, Gu XY, Gao SX (2011) Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J Colloid Interf Sci 361:247–251
Zhou QX (1995) Ecology of combined pollution. China Environmental Science Press, Beijing
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China as a general project (grant no. 40901259) and a key project (grant no. 21037002), and by OPCW support (grant no. AC/19097).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Jean-Paul Schwitzguébel
Rights and permissions
About this article
Cite this article
Bao, Yy., Wan, Y., Zhou, Qx. et al. Competitive adsorption and desorption of oxytetracycline and cadmium with different input loadings on cinnamon soil. J Soils Sediments 13, 364–374 (2013). https://doi.org/10.1007/s11368-012-0600-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-012-0600-3