Abstract
Background, aim, and scope
The cause for this position paper is the impression that risk assessors consider primarily the concentration of free metal ions dissolved in solution controlling metal bioavailability in aquatic systems. Aiming at a more realistic risk assessment of metals, bioavailability has to be discussed under the scope of main uptake routes of metals to organisms.
Materials and methods
On the basis of a review on the literature relating to bioavailability approaches, this work discusses the incorporation of metal bioavailability into the risk assessment of metals in the context of metal exposure.
Results
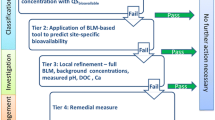
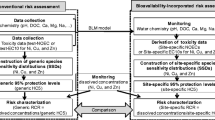
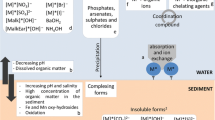
The biotic ligand model (BLM) and the concept of sulfide bound metals described by the ratio of simultaneously extracted metals and acid volatile sulfide concept (AVS) have been developed to consider the bioavailability of metals. Both approaches assume that the free ion concentration is the most relevant exposure pathway. However, apart from geochemical conditions, which control free metal concentration, bioavailability is additionally a result of contaminant/particle interaction and of organisms' activity. Asking for the relevant exposure pathways for inorganic metals to organisms, the compartments' water and sediment have been evaluated and also the importance of contaminated food.
Discussion
We present a conceptual model of the main processes and sources for uptake of trace metals at a biological membrane. On the basis of this model, we have to consider free metal ions, metal complexes, and particle-bound metals. The BLM approach has been proposed for use in European Union risk assessments. However, the BLM provides a means to predict ecotoxicological effect of metals in the environment, but at present assumes that total significant uptake is from the dissolved phase. It is apparent that dietary accumulation of metals is at least as important as metal uptake from the aqueous phase and in many cases dominates metal accumulation.
Conclusions
We found evidence in literature that uptake occurs via the dissolved phase, metal complexes, dietary, and particle-bound metals. In this regard, the AVS model, which considers only sedimentary metals in anoxic sediments, was more effective in predicting metal concentrations in pore waters than sediment toxicity in general.
Recommendations and perspectives
Models will be improved by incorporating chronic metal effects rather than the binding to ligands. The most important for a risk assessment is a broad understanding of the relative importance of different uptake routes and the differential toxicity of metals accumulated by organisms with diverse feeding behavior.


Similar content being viewed by others
References
Ahlf W, Förstner U (2001) Managing contaminated sediments—I. Improving chemical and biological criteria. J Soils Sediments 1:30–36
Allen HE, Janssen CR (2006) Incorporating bioavailability into criteria for metals. In: Twardowska I, Allen HE, Haggblom MH (eds.) Soil and water pollution monitoring, protection and remediation. Springer, Netherlands, pp 3–23
Ankley GT, Di Toro DM, Hansen DJ, Berry WJ (1996) Technical basis and proposal for deriving sediment quality criteria for metals. Environ Toxicol Chem 15(12):2056–2066
Batley GE, Apte SC, Stauber JL (2004) Speciation and bioavailability of trace metals in water, Progress since 1982. Austral J Chem 57:903–919
Berry WJ, Cantwell MG, Edwards PA, Serbst JR, Hansen DJ (1999) Predicting toxicity of sediments spiked with silver. Environ Toxicol Chem 18:40–48
Burton ED, Phillips IR, Hawker DW (2005) Geochemical partitioning of copper, lead, and zinc in benthic, estuarine sediment profiles. J Environ Qual 34:263–273
Calace N, Petronio BM, Pietroletti M (2006) Metal bioavailability. How does its significance change in the time? Annali di Chimica 96:131–136
Camusso M, Gasparella A (2006) Measuring bioavailable trace metals from freshwater sediments by diffusive gradients in thin films (DGT) in monitoring procedures for quality assessment. Annali di Chimica 96:205–213
Crane M (2003) Proposed development of Sediment Quality Guidelines under the European Water Framework Directive: a critique. Toxicol Lett 142:195–206
Croteau MN, Luoma SN (2007) Characterizing dissolved Cu and Cd uptake in terms of the biotic ligand and biodynamics using enriched stable isotopes. Environ Sci Technol 41:3140–3145
Croteau MN, Luoma SN, Stewart AR (2005) Trophic transfer of metals along freshwater food webs, evidence of cadmium biomagnification in nature. Limnol Oceanogr 50:1511–1519
Croteau MN, Luoma SN, Pellet B (2007) Determining metal assimilation efficiency in aquatic invertebrates using enriched stable metal isotope tracers. Aquatic Toxicology 83:116–125
De Schamphelaere KAC, Janssen CR (2002) A biotic ligand model predicting acute copper toxicity for Daphnia magna: effects of calcium, magnesium, sodium and pH. Environl Sci Technol 36:48–54
De Schamphelaere KAC, Janssen CR (2004a) Development and field validation of a biotic ligand model predicting chronic copper toxicity to Daphnia magna. Environ Toxicol Chem 23:1365–1375
De Schamphelaere KAC, Janssen CR (2004b) Effects of dissolved organic carbon concentration and source, pH, and water hardness on chronic toxicity of copper to Daphnia magna. Environ Toxicol Chem 23:115–1122
Deleebeeck NME, Muyssen BTA, De Laender F, Janssen CR, De Schamphelaere KAC (2007) Comparison of nickel toxicity to cladocerans in soft versus hard surface waters. Aquat Toxicol 84:223–235
Deleebeeck NME, De Schamphelaere KAC, Janssen CR (2008) A novel method predicting nickel bioavailability and toxicity to Daphnia magna in artificial and natural waters. Environ Toxicol Chem 27:2097–2108
Di Toro DM, Allen HE, Bergman HL, Meyer JS, Paquin PR (2001) Biotic ligand model of the acute toxicity of metals. 1. Technical basis. Environ Toxicol Chem 20:2383–2396
Di Toro DM, McGrath JA, Hansen DJ, Berry WJ, Paquin PR, Mathew R (2005) Predicting sediment metal toxicity using a sediment biotic ligand model. Methodology and initial application. Environ Toxicol Chem 24:2410–2427
Erickson RJ, Benoit DA, Mattson VR, Nelson HP Jr, Leonard EN (1996) The effects of water chemistry on the toxicity of copper to fathead minnows. Environ Toxicol Chem 15(2):181–193
Escher BI, Hermens JL (2004) Internal exposure, linking bioavailability to effects. Environ Sci Technol 38:455A–462A
Europäische Gemeinschaft (EG), 2006/Draft2009. Verordnung (EG) Nr. 1907/2006 des Europäischen Parlaments und des Rates vom 18. Dezember 2006 zur Registrierung, Bewertung, Zulassung und Beschränkung chemischer Stoffe (REACH), zur Schaffung einer Europäischen Agentur für chemische Stoffe, zur Änderung der Richtlinie 1999/45/EG und zur Aufhebung der Verordnung (EWG) Nr. 793/93 des Rates, der Verordnung (EG) Nr. 1488/94 der Kommission, der Richtlinie 76/769/EWG des Rates sowie der Richtlinien 91/155/EWG, 93/67/EWG, 93/105/EG und 2000/21/EG der Kommission. Amtsblatt der Europäischen Union, Nr. L 396/1. 851 S
Fairbrother A, Wenstel R, Sappington K, Wood W (2007) Framework for metals risk assessment. Ecotox Environl Saf 68:145–227
Ferreira D, Tousset N, Ridame C, Tusseau-Vuillemin MH (2008) More than inorganic copper is bioavailable to aquatic mosses at environmentally relevant concentrations. Environ Toxicol Chem 27:2108–2116
Förstner U (2009) Sediments and priority substances in river basins—new directive 2008/105/EC; sediment issues in management plans. J Soils Sediments 9(2):89–93
Griscom SB, Fisher NS, Aller RC, Lee BG (2002) Effects of gut chemistry in marine bivalves on the assimilation of metals from ingested sediment particles. J Mar Res 60:101–120
Guo LD, Santschi PH, Ray SM (2002) Metal partitioning between colloidal and dissolved phases and its relation with bioavailability to American oysters. Mar Environ Res 54:49–64
Hare L, Tessier A, Warren L (2001) Cadmium accumulation by invertebrates living at the sediment–water interface. Environ Toxicol Chem 20:880–889
Hassler CS, Slaveykova VI, Wilkinson KJ (2004) Some fundamental (and often overlooked) considerations underlying the free ion activity and biotic ligand models. Environ Toxicol Chem 23:283–291
Hsu P, Matthäi A, Heise S, Ahlf W (2007) Seasonal variation of sediment toxicity in river basins of Dommel and Elbe. Environ Pollut 148:817–823
Keung CF, Guo F, Qian PY, Wang WX (2008) Influences of metal–ligand complexes on the cadmium and zinc biokinetics in the marine bacterium, Bacillus firmus. Environ Toxicol Chem 27:131–137
Lee BG, Lee JS, Luoma SN, Choi HJ, Koh CH (2000) Influence of acid volatile sulphide and metal concentrations on metal bioavailability to marine invertebrates in contaminated sediments. Environ Sci Technol 34:4517–4523
Liss W, Ahlf W (1997) Evidence from whole sediment, pore water, and elutriate testing in toxicity assessment of contaminated sediments. Ecotoxicol Environ Saf 36:140–147
Liu JC, Yan CL, Macnair MR, Hu J, Li YH (2007) Vertical distribution of acid-volatile sulphide and simultaneously extracted metals in mangrove sediments from the Jiulong River Estuary, Fujian, China. Environ Sci Pollut Res 14:345–349
Lorenzo JI, Beiras R, Mubiana VK, Blust R (2005) Copper uptake by Mytilus edulis in the presence of humic acids. Environ Toxicol Chem 24:973–980
Lu XQ, Werner I, Young TM (2005) Geochemistry and bioavailability of metals in sediments from northern San Francisco Bay. Environ Int 31:593–602
Luoma SN, Rainbow PS (2005) Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ Sci Technol 39:1921–1931
Ma H, Kim SD, Cha DK, Allen HE (1999) Effect of kinetics of complexation by humic acid on toxicity of copper to Ceriodaphnia dubia. Environ Toxicol Chem 18(5):828–837
Martin AJ, Goldblatt R (2007) Speciation, behavior, and bioavailability of copper downstream of a mine-impacted lake. Environ Toxicol Chem 26(12):2594–2603
McGeer JC, Szebedinszky C, McDonald DG, Wood CM (2002) The role of dissolved organic carbon in moderating the bioavailability and toxicity of Cu to rainbow trout during chronic waterborne exposure. Comp Biochem Phys 133:147–160
Meyer JS (2002) The utility of the terms “bioavailability” and “bioavailable fraction” for metals. Mar Environ Res 53:417–423
MERAG (2007) Incorporation of bioavailability for water, soils and sediments. Metals Risk Assessment Guidance (MERAG) Fact Sheet 05. ICMM, London, 23 pp. http://www.euras.be/assets/files/MERAG/MERAG%20FS%2005%20Jan%2007.pdf, 12.05.2009
Offermann K, Matthäi A, Ahlf W (2009) Assessing the importance of dietborne cadmium and particle characteristics on bioavailability and bioaccumulation in the nematode Caenorhabditis elegans. Environ Toxicol Chem 28(6):1149–1158. doi:10.1897/08-272.1
PeijnenburgWJGM ZM, Vijver MG (2007) Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicol Environ Saf 67:163–179
Rainbow PS (2007) Trace metal bioaccumulation: models, metabolic availability and toxicity. Environ Int 33:576–582
Rainbow PS, Wang WX (2001) Comparative assimilation of Cd, Cr, Se, and Zn by the barnacle Elminius modestus from phytoplankton and zooplankton diets. Marine Ecol-Prog Ser 218:239–248
Reiley MC (2007) Science, policy, and trends of metals risk assessment at EPA. How understanding metals bioavailability has changed metals risk assessment at US EPA. Aquat Toxicol 84:292–298
Roditi HA, Fisher NS, Sanudo-Wilhelmy SA (2000) Field testing a metal bioaccumulation model for zebra mussels. Environ Sci Technol 34:2817–2825
Sanchez-Marin P, Lorenzo JI, Blust R, Beiras R (2007) Humic acids increase dissolved lead bioavailability for marine invertebrates. Environ Sci Technol 41:5679–5684
Santore C, Di Toro DM, Paquin PR, Allen HE, Meyer JS (2001) Biotic ligand model of the acute toxicity of metals. 2. Application to acute copper toxicity in freshwater fish and Daphnia. Environ Toxicol Chem 20:2397–2402
Schlekat CE, Luoma SN (2000) You are what you eat, incorporating dietary metals uptake into environmental quality guidelines for aquatic ecosystems. SETAC Globe 1(2):38–39
Selck H, Forbes VE (2004) The relative importance of water and diet foruptake and subcellular distribution of cadmium in the deposit-feeding polychaete. Capitella sp. I. Mar Environ Res 57:261–279
Simpson SL, Batley GE (2007) Predicting metal toxicity in sediments: a critique of current approaches. Integr Environ Assess Manag 3(1):18–31
Simpson SL, King CK (2005) Exposure-pathway models explain causality in whole-sediment toxicity tests. Environ Sci Technol 39:837–843
Sofyan AS, Shaw JR, Birge WJ (2006) Metal trophic transfer from algae to cladocerans and the relative importance of dietary metal exposure. Environ Toxicol Chem 25:1034–1041
Sundelin B, Eriksson AK (2001) Mobility and bioavailability of trace metals in sulfidic coastal sediments. Environ Toxicol Chem 20:748–756
Twining BS, Fisher NS (2004) Trophic transfer of trace metals from protozoa to mesozooplankton. Limnol Oceanogr 49:28–39
US EPA (Environmental Protection Agency) (2003) Guidance for the Development of Ecological Soil Screening Levels. Office of Solid Waste and Emergency Response, Washington, DC (OSWER Directive 92857-55)
US EPA (Environmental Protection Agency) (2004) Draft aquatic life water quality criteria for selenium. Office of Water. EPA-822-D-04-001
Van Leeuwen HP, Pinheiro JP (2001) Speciation dynamics and bioavailability of metals. Exploration of the case of two uptake routes. Pure Appl Chem 73:39–44
Vijver MG, Van Gestel CAM, Lanno RP, Van Straalen NM, Peijnenburg WJGM (2004) Internal metal sequestration and its ecotoxicological relevance. A review. Environ Sci Technol 8:4705–4712
Wang WX, Guo LD (2000a) Influences of natural colloids on metal bioavailability to two marine bivalves. Environ Sci Technol 34:4571–4576
Wang WX, Guo LD (2000b) Bioavailability of colloid-bound Cd, Cr, and Zn to marine plankton. Mar Ecol Prog Ser 202:41–49
Wang WX, Ke C (2002) Dominance of dietary intake of cadmium and zinc by two marine predatory gastropods. Aquat Toxicol 56:153–165
Worms I, Simon DF, Hassler CS, Wilkinson KJ (2006) Bioavailability of trace metals to aquatic microorganisms, importance of chemical, biological and physical processes on biouptake. Biochimie 88:1721–1731
Xu Y, Wang W-X (2002) Exposure and potential food chain transfer factor of Cd, Se, and Zn in marine fish Lutjanus argentimaculatus. Mar Ecol Prog Ser 238:173–186
Yan QL, Wang WX (2002) Metal exposure and bioavailability to a marine deposit-feeding sipuncula, Sipunculus nudus. Environ Sci Technol 36:40–47
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Henner Hollert
Rights and permissions
About this article
Cite this article
Ahlf, W., Drost, W. & Heise, S. Incorporation of metal bioavailability into regulatory frameworks—metal exposure in water and sediment. J Soils Sediments 9, 411–419 (2009). https://doi.org/10.1007/s11368-009-0109-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-009-0109-6