Abstract
Purpose
The present study aims to understand the influence of rearing practices and the contributions of production phases of fish farming to their environmental impacts and determine which practices and technical characteristics can best improve the farms’ environmental performance. Another objective is to identify the influence of variability in farming practices on the environmental performances of sea cage aquaculture farms of sea bass and sea bream in Tunisia by using principal component analysis (PCA) and hierarchical clustering on principal components (HCPC) methods and then combining the classification with life cycle assessment (LCA).
Methods
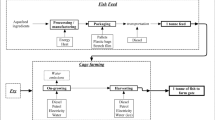
The approach consisted of three major steps: (i) of the 24 aquaculture farms in Tunisia, 18 were selected which follow intensive rearing practices in sea cages of European sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata) and then a typology was developed to classify the studied farms into rearing practice groups using HCPC; (ii) LCA was performed on each aquaculture farm and (iii) mean impacts and contributions of production phases were calculated for each group of farms. Impact categories included acidification, eutrophication, global warming, land occupation, total cumulative energy demand and net primary production use.
Results and discussion
Results revealed high correlation between rearing practices and impacts. The feed-conversion ratio (FCR), water column depth under the cages and cage size had the greatest influence on impact intensity. Rearing practices and fish feed were the greatest contributors to the impacts studied due to the production of fish meal and oil and the low efficiency of feed use, which generated large amounts of nitrogen and phosphorus emissions. It is necessary to optimise the diet formulation and to follow better feeding strategies to lower the FCR and improve farm performance. Water column depth greatly influenced the farms’ environmental performance due to the increase in waste dispersion at deeper depths, while shallow depths resulted in accumulation of organic matter and degradation of water quality. Cage size influences environmental performances of aquaculture farms. Thus, from an environmental viewpoint, decision makers should grant licences for farms in deeper water with larger cages and encourage them to improve their FCRs.
Conclusions
This study is the first attempt to combine the HCPC method and the LCA framework to study the environmental performance of aquacultural activity. The typology developed captures the variability among farms because it considers several farm characteristics in the classification. The LCA demonstrated that technical parameters in need of improvement are related to the technical expertise of farm managers and workers and to the location of the farm.







Similar content being viewed by others
References
Abdi H, Williams LJ (2010) Principal component analysis. Wiley Interdiscip Rev Comput Stat 2:433–459
Abdou K, Aubin J, Romdhane MS et al (2017) Environmental assessment of seabass (Dicentrarchus labrax) and seabream (Sparus aurata) farming from a life cycle perspective: a case study of a Tunisian aquaculture farm. Aquaculture 471:204–212
Aubin J (2013) Life cycle assessment as applied to environmental choices regarding farmed or wild-caught fish. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour
Aubin J, Baruthio A, Mungkung R, Lazard J (2015) Environmental performance of brackish water polyculture system from a life cycle perspective: a Filipino case study. Aquaculture 435:217–227
Aubin J, Papatryphon E, van der Werf HMG, Chatzifotis S (2009) Assessment of the environmental impact of carnivorous finfish production systems using life cycle assessment. J Clean Prod 17:354–361
Borja Á, Rodríguez JG, Black K et al (2009) Assessing the suitability of a range of benthic indices in the evaluation of environmental impact of fin and shellfish aquaculture located in sites across Europe. Aquaculture 293:231–240
Bureau DP, Gunther SJ, Cho CY (2003) Chemical composition and preliminary theoretical estimates of waste outputs of rainbow trout reared in commercial cage culture operations in Ontario. North Am J Aquac 65:33–38
Canario AV, Condeca J, Power D, Ingleton P (1998) The effect of stocking density on growth in the gilthead sea-bream, Sparus aurata (L.) Aquac Res 29:177–181
Chen X, Samson E, Tocqueville A, Aubin J (2015) Environmental assessment of trout farming in France by life cycle assessment: using bootstrapped principal component analysis to better define system classification. J Clean Prod 87:87–95
Cho CY, Kaushik SJ (1990) Nutritional energetics in fish: energy and protein utilization in rainbow trout (Salmo Gairdneri). World Rev Nutr Diet 61:132–172
Cromey CJ, Nickell TD, Black KD (2002) DEPOMOD—modelling the deposition and biological effects of waste solids from marine cage farms. Aquaculture 214:211–239
Consultants P (1997) SimaPro 2 method. Database manual. Pré Consultants B.V, Amersfoort
DGPA (2014) Annuaire des statistiques des pêches en Tunisie. Ministère de l’Agriculture, Tunisie
Dias J, Conceição LE, Ribeiro AR et al (2009) Practical diet with low fish-derived protein is able to sustain growth performance in gilthead seabream (Sparus aurata) during the grow-out phase. Aquaculture 293:255–262
Di Marco P, Priori A, Finoia MG et al (2008) Physiological responses of European sea bass Dicentrarchus labrax to different stocking densities and acute stress challenge. Aquaculture 275:319–328
Ellingsen H, Aanondsen SA (2006) Environmental impacts of wild caught cod and farmed Salmon-a comparison with chicken. Int J Life Cycle Assess 11:60–65
European Commission (2010) International reference life cycle data system (ILCD) handbook—general guide for life cycle assessment. Joint Research Centre. Institute for Environment and Sustainability, Luxembourg
FAO (Food and Agriculture Organization of the United Nations) (2016) The state of world fisheries and aquaculture: contributing to food security and nutrition for all. Food and Agriculture Organization of the United Nations, Rome
Fréon P, Avadí A, Vinatea Chavez RA, Iriarte Ahón F (2014) Life cycle assessment of the Peruvian industrial anchoveta fleet: boundary setting in life cycle inventory analyses of complex and plural means of production. Int J Life Cycle Assess 19:1068–1086
Gauthier TD (2001) Detecting trends using Spearman’s rank correlation coefficient. Environ Forensic 2:359–362
Guinée JB, Gorrée M, Heijungs R et al (2002) Handbook on life cycle assessment operational guide to the ISO standards. Dordr Kluwer Acad Publ 704
Helmes RJ, Huijbregts MA, Henderson AD, Jolliet O (2012) Spatially explicit fate factors of phosphorous emissions to freshwater at the global scale. Int J Life Cycle Assess 17:646–654
Henriksson PJG, Heijungs R, Dao HM et al (2015) Product carbon footprints and their uncertainties in comparative decision contexts. PLoS One 10:e0121221
Huijbregts M (1999) Life-cycle impact assessment of acidifying and eutrophying air pollutants. Calculation of equivalency factors with RAINS-LCA. Interfaculty Department of Environmental Science, Faculty of Environmental Science, University of Amsterdam, Amsterdam
Huntingford FA, Adams C, Braithwaite VA et al (2006) Current issues in fish welfare. J Fish Biol 68:332–372
Iguchi K, Ogawa K, Nagae M, Ito F (2003) The influence of rearing density on stress response and disease susceptibility of ayu (Plecoglossus altivelis). Aquaculture 220:515–523
IPCC (2014) Fifth Assessment Report (AR5) Climate Change 2014, Synthesis Report. Intergovernmental Panel for Climate Change. Available from: http://www.ipcc.ch/. Accessed 10 Jan 2017
ISO (The International Organization for Standardization) (2006a) Environmental management—life cycle assessment—requirements and guidelines. ISO 14044, ISO, Geneva
ISO (The International Organization for Standardization) (2006b) Environmental management—life cycle assessment—principles and framework. ISO 14044, ISO, Geneva
Jerbi MA, Aubin J, Garnaoui K et al (2012) Life cycle assessment (LCA) of two rearing techniques of sea bass (Dicentrarchus labrax). Aquac Eng 46:1–9
Kristiansen TS, Fernö A, Holm JC et al (2004) Swimming behaviour as an indicator of low growth rate and impaired welfare in Atlantic halibut (Hippoglossus hippoglossus L.) reared at three stocking densities. Aquaculture 230:137–151
Lazard J, Baruthio A, Mathé S et al (2010) Aquaculture system diversity and sustainable development: fish farms and their representation. Aquat Living Resour 23:187–198
Lefrançois C, Claireaux G, Mercier C, Aubin J (2001) Effect of density on the routine metabolic expenditure of farmed rainbow trout (Oncorhynchus mykiss). Aquaculture 195:269–277
Lê S, Josse J, Husson F (2008) FactoMineR: a package for multivariate analysis. J Stat Softw 25:1–18
Montero D, Izquierdo M, Tort L et al (1999) High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiol Biochem 20:53–60
Mungkung R, Aubin J, Prihadi TH et al (2013) Life cycle assessment for environmentally sustainable aquaculture management: a case study of combined aquaculture systems for carp and tilapia. J Clean Prod 57:249–256
Naylor RL, Goldburg RJ, Primavera JH et al (2000) Effect of aquaculture on world fish supplies. Nature 405:1017–1024
North BP, Turnbull JF, Ellis T et al (2006) The impact of stocking density on the welfare of rainbow trout (Oncorhynchus mykiss). Aquaculture 255:466–479
Papatryphon E, Petit J, Kaushik SJ, van der Werf HM (2004) Environmental impact assessment of salmonid feeds using life cycle assessment (LCA). AMBIO J Hum Environ 33:316–323
Pauly D, Christensen V (1995) Primary production required to sustain global fisheries. Nature 374:255–257
Procarione LS, Barry TP, Malison JA (1999) Effects of high rearing densities and loading rates on the growth and stress responses of juvenile rainbow trout. North Am J Aquac 61:91–96
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Read P, Fernandes T (2003) Management of environmental impacts of marine aquaculture in Europe. Aquaculture 226:139–163
Richardson CJ, Qian SS (1999) Long-term phosphorus assimilative capacity in freshwater wetlands: a new paradigm for sustaining ecosystem structure and function. Environ Sci Technol 33:1545–1551
Rowland SJ, Mifsud C, Nixon M, Boyd P (2006) Effects of stocking density on the performance of the Australian freshwater silver perch (Bidyanus bidyanus) in cages. Aquaculture 253:301–308
Sammouth S, d’Orbcastel ER, Gasset E et al (2009) The effect of density on sea bass (Dicentrarchus labrax) performance in a tank-based recirculating system. Aquac Eng 40:72–78
Schram E, Van der Heul J, Kamstra A, Verdegem M (2006) Stocking density-dependent growth of dover sole (Solea solea). Aquaculture 252:339–347
Spearman C (1904) The proof and measurement of association between two things. Am J Psychol 15:72–101
Tacon AG (2005) State of information on salmon aquaculture feed and the environment. Rep WWF Salmon Aquac Dialogue 1–80
Tovar A, Moreno C, Manuel-Vez MP, García-Vargas M (2000) Environmental implications of intensive marine aquaculture in earthen ponds. Mar Pollut Bull 40:981–988
Turnbull J, Bell A, Adams C et al (2005) Stocking density and welfare of cage farmed Atlantic salmon: application of a multivariate analysis. Aquaculture 243:121–132
Vazzana M, Cammarata M, Cooper E, Parrinello N (2002) Confinement stress in sea bass (Dicentrarchus labrax) depresses peritoneal leukocyte cytotoxicity. Aquaculture 210:231–243
Acknowledgements
The authors would like to acknowledge valuable financial support from the ‘Institut de Recherche pour le Développement’ (JEAI GAMBAS project). This study was also partially funded by ‘LabexMer’.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angel Avadí
Electronic supplementary material
ESM 1
(DOCX 30 kb).
Rights and permissions
About this article
Cite this article
Abdou, K., Ben Rais Lasram, F., Romdhane, M.S. et al. Rearing performances and environmental assessment of sea cage farming in Tunisia using life cycle assessment (LCA) combined with PCA and HCPC. Int J Life Cycle Assess 23, 1049–1062 (2018). https://doi.org/10.1007/s11367-017-1339-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-017-1339-2