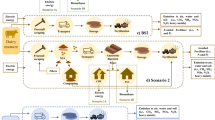
Abstract
Purpose
Integrated agriculture and aquaculture (IAA), as typified by the mulberry dike-pond system (DPS) of the Pearl River delta of southern China, is often cited as an example of sustainable intensified production due to its characteristic closed loop recycling of nutrients. In this study, we tackle two issues that have been hardly addressed in previous analyses of aquaculture production, greenhouse gas emissions (GHGe) from the pond and the role of labour.
Methods
Previous assessments led us to revisit the sustainability of the DPS system as a model for a well-studied IAA system using a life cycle assessment (LCA) methodology. Our study quantifies on-farm CH4 and N2O emissions and indirect emissions embedded in inputs, using the global warming potential (GWP) metric. To model the indirect impact of the high labour requirements of the system, a simple methodology based on metabolizable energy requirements is proposed.
Results and discussion
Our GHGe assessment suggests that using fish ponds to treat organic waste results in higher net emissions than alternative waste processing options (e.g. composting), even when the co-production of fish is accounted for. The majority of total system GWP100 (97 %) can be attributed to methane from the fertilised ponds. Food required to meet labour requirements plays an important role, from 11 to 22 % of total environmental impact.
Conclusions
Methane from semi-intensive ponds fertilised with organic waste appears to be a significant source of GWP, calling into question the environmental sustainability of IAA systems such as the mulberry DPS. Improving sustainability in such systems will require better understanding of GHGe from waste-fed aquaculture ponds, notably with respect to on-farm N2O and CH4.




Similar content being viewed by others
References
Amlinger F, Peyr S, Cuhls C (2008) Green house gas emissions from composting and mechanical biological treatment. Waste Manag Res 26:47–60
Astudillo MF, Thalwitz G, Vollrath F (2014) Life cycle assessment of Indian silk. J Clean Prod 81:158–167
Aubin J, Papatryphon E, Van der Werf HMG et al (2006) Characterisation of the environmental impact of a turbot (Scophthalmus maximus) re-circulating production system using Life Cycle Assessment. Aquaculture 261:1259–1268
Aubin J, Papatryphon E, van der Werf HMG, Chatzifotis S (2009) Assessment of the environmental impact of carnivorous finfish production systems using life cycle assessment. J Clean Prod 17:354–361
Bastviken D, Cole JJ, Pace ML, Van de Bogert MC (2008) Fates of methane from different lake habitats: connecting whole-lake budgets and CH4 emissions. J Geophys Res 113:G02024. doi:10.1029/2007JG000608
Blackburn TH (1987) Role and impact of anaerobic microbial processes in aquatic systems. In: Moriarty DJW, Pullin RSV (eds) Detritus Microb. Ecol. Aquac. ICLARM, Manila, pp 32–53
Bosma R, Anh PT, Potting J (2011) Life cycle assessment of intensive striped catfish farming in the Mekong Delta for screening hotspots as input to environmental policy and research agenda. Int J Life Cycle Assess 16:903–915
Bostock J, McAndrew B, Richards R et al (2010) Aquaculture: global status and trends. Philos Trans R Soc Lond B Biol Sci 365:2897–2912
Bussmann I, Damm E, Schlüter M, Wessels M (2013) Fate of methane bubbles released by pockmarks in Lake Constance. Biogeochemistry 112:613–623
Cai Z, Shan Y, Xu H (2007) Effects of nitrogen fertilization on CH4 emissions from rice fields. Soil Sci Plant Nutr 53:353–361
Cao L, Naylor R, Henriksson P et al (2015) China’s aquaculture and the world’s wild fisheries. Science 347:11–13
Chang WYB, Ouyang H (1988) Dynamics of dissolved oxygen and vertical circulation in fish ponds. Aquaculture 74:263–276
Chen GQ, Jiang MM, Chen B et al (2006a) Emergy analysis of Chinese agriculture. Agric Ecosyst Environ 115:161–173
Chen M, Jin P, Huang L, Lu X (2006b) Emergy analysis of mulberry silkworm ecosystem in China. Chin J Appl Ecol 17:233–236
Coche AG, Muir JF, Laughlin T (1996) Simple methods for aquaculture: management for freshwater fish culture ponds and water practices. FAO, Rome
CSRTI (2013) Annual report 2012-2013. Mysore
Datta A, Nayak DR, Sinhababu DP, Adhya TK (2009) Methane and nitrous oxide emissions from an integrated rainfed rice–fish farming system of Eastern India. Agric Ecosyst Environ 129:228–237
Dazhong W, Pimentel D (1986) Seventeenth century organic agriculture in China: II. Energy flows through an agroecosystem in Jiaxing region. Hum Ecol 14:15–28
De Klein C, Novoa RS, Ogle S et al (2006) N2O emissions from managed soils, and CO2 emissions from lime and urea application. 2006 IPCC Guidel. Natl. Greenh. gas Invent, pp 1–54
Detweiler AM, Bebout BM, Frisbee AE et al (2014) Characterization of methane flux from photosynthetic oxidation ponds in a wastewater treatment plant. Water Sci Technol 70:980
Deutzmann JS, Schink B (2011) Anaerobic oxidation of methane in sediments of Lake Constance, an oligotrophic freshwater lake. Appl Environ Microbiol 77:4429–4436
Diana JS, Lin CK, Schneeberger PJ (1991) Relationships among nutrient inputs, water nutrient concentrations, primary production, and yield of Oreochromis niloticus in ponds. Aquaculture 92:323–341
Du SF, Wang HJ, Zhang B et al (2014) China in the period of transition from scarcity and extensive undernutrition to emerging nutrition-related non-communicable diseases, 1949-1992. Obes Rev 15(Suppl 1):8–15
Edwards P (1993) Environmental issues in integrated agriculture-aquaculture and wastewater-fed culture systems. In: Pullin RSV, Rosenthal H, Maclean JL (eds) Environ. Aquac. Dev. Ctries. ICLARM, Manila, pp 139–170
Ettwig KF, Butler MK, Le Paslier D et al (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548
European Commission (2010) International reference life cycle data system (ILCD) handbook - general guide on LCA - general guide for life cycle assessment. doi:10.2788/38479
FAO (1972) Food composition table for East Asia. Rome, Italy
FAO (2004) Human energy requirements. FAO, Rome
FAO Faostat. In: 2014. faostat.fao.org. Accessed 20 Sep 2014
Ferrón S, Ortega T, Gómez-Parra A, Forja JM (2007) Seasonal study of dissolved CH4, CO2 and N2O in a shallow tidal system of the bay of Cádiz (SW Spain). J Mar Syst 66:244–257
Frei M, Becker K (2005) Integrated rice–fish production and methane emission under greenhouse conditions. Agric Ecosyst Environ 107:51–56
Galloway JN, Townsend AR, Erisman JW et al (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Garnett T, Appleby MC, Balmford A et al (2013) Sustainable intensification in agriculture: premises and policies. Science 341:33–34
Giampietro M (2006) Comments on “The Energetic Metabolism of the European Union and the United States” by Haberl and Colleagues Theoretical and Practical Considerations on Energy Analysis. J Ind Ecol 10:173–185
Giampietro M, Pimentel D (1990) Assessment of the energetics of human labor. Agric Ecosyst Environ 32:257–272
Godfray HCJ, Beddington JR, Crute IR et al (2010) Food security: the challenge of feeding 9 billion people. Science 327:812–818
Gross A, Boyd CE, Wood CWW (2000) Nitrogen transformations and balance in channel catfish ponds. Aquac Eng 24:1–14
Held RB, Zhang Q, Mihelcic JR (2013) Quantification of human and embodied energy of improved water provided by source and household interventions. J Clean Prod 60:83–92
Henriksson PJG, Guinée JB, Heijungs R et al (2013) A protocol for horizontal averaging of unit process data—including estimates for uncertainty. Int J Life Cycle Assess 19:429–436
Henriksson PJG, Zhang W, Nahid SA et al (2014) Final LCA case study report - annex
Hinshaw SE, Dahlgren RA (2013) Dissolved nitrous oxide concentrations and fluxes from the eutrophic San Joaquin River, California. Environ Sci Technol 47:1313–1322
Hu Z, Lee JW, Chandran K et al (2013) Nitrogen transformations in intensive aquaculture system and its implication to climate change through nitrous oxide emission. Bioresour Technol 130:314–320
Huijbregts MAJ, Hellweg S, Frischknecht R et al (2010) Cumulative energy demand as predictor for the environmental burden of commodity production. Environ Sci Technol 44:2189–2196
Kampschreur MJ, Temmink H, Kleerebezem R et al (2009) Nitrous oxide emission during wastewater treatment. Water Res 43:4093–4103
Lambin EF, Meyfroidt P (2011) Global land use change, economic globalization, and the looming land scarcity. Proc Natl Acad Sci U S A 108:3465–3472
Lin GCS (1997) Transformation of a rural economy in the Zhujiang Delta. China Q 149:56–80
Lo CP (1996) Environmental impact on the development of agricultural technology in China: the case of the dike-pond (‘jitang’) system of integrated agriculture-aquaculture in the Zhujiang Delta of China. Agric Ecosyst Environ 60:183–195
Lu L, Tang Y, Xie J, Yuan Y (2009) The role of marginal agricultural land-based mulberry planting in biomass energy production. Renew Energy 34:1789–1794
Makkar HPS, Tran G, Heuzé V, Ankers P (2014) State-of-the-art on use of insects as animal feed. Anim Feed Sci Technol 197:1–33
Naylor RL, Goldburg RJ, Primavera JH et al (2000) Effect of aquaculture on world fish supplies. Nature 405:1017–1024
Ndong R, Montrejaud-Vignoles M, Saint Girons O et al (2009) Life cycle assessment of biofuels from Jatropha curcas in West Africa: a field study. GCB Bioenergy 1:197–210
Nguyen TLT, Hermansen JE, Mogensen L (2010) Environmental consequences of different beef production systems in the EU. J Clean Prod 18:756–766
Nhan DK, Verdegem MCJ, Binh NT et al (2008a) Economic and nutrient discharge tradeoffs of excreta-fed aquaculture in the Mekong Delta, Vietnam. Agric Ecosyst Environ 124:259–269
Nhan DK, Verdegem MCJ, Milstein A, Verreth JAV (2008b) Water and nutrient budgets of ponds in integrated agriculture-aquaculture systems in the Mekong Delta, Vietnam. Aquac Res 39:1216–1228
Odum HT (1988) Self-organization, transformity, and information. Science 242:1132–1139
Oláh J, Sinha VRP, Ayyappan S et al (1986) Primary production and fish yields in fish ponds under different management practices. Aquaculture 58:111–122
Panneer Selvam B, Natchimuthu S, Arunachalam L, Bastviken D (2014) Methane and carbon dioxide emissions from inland waters in India - implications for large scale greenhouse gas balances. Glob Chang Biol 2:3397–3407
Pelletier N, Tyedmers P (2010) Life cycle assessment of frozen tilapia fillets from Indonesian lake-based and pond-based intensive aquaculture systems. J Ind Ecol 14:467–481
Pelletier N, Audsley E, Brodt S et al (2011) Energy intensity of agriculture and food systems. Annu Rev Environ Resour 36:223–246
Phong LT, de Boer IJM, Udo HMJ (2011) Life cycle assessment of food production in integrated agriculture–aquaculture systems of the Mekong Delta. Livest Sci 139:80–90
Pimentel D, Hurd LE, Bellotti AC et al (1973) Food production and the energy crisis. Science 182:443–449
Riise JC, Roos N (1997) Benthic metabolism and the effects of bioturbation in a fertilised polyculture fish pond in northeast Thailand. Aquaculture 150:45–62
Ruddle K (1985) Rural reforms and household economies in the dike-pond area of the Zhujiang Delta, China. Bull Natl Museum Ethnol 10:1145–1174
Ruddle K, Christensen V (1993) An energy flow model of the mulberry dike-carp pond farming system of the Zhujiang Delta, Guandong Province, China. In: Christensen V, Pauly D (eds) Trophic Model. Aquat. Ecosyst. ICLARM, Manila, pp 48–55
Ruddle K, Zhong G (1988) Integrated agriculture-aquaculture in South China: the dike-pond system in the Zhujiang Delta. Cambridge University Press, New York
Ruddle K, Hanzeng D, Guozhao L (1986) Energy exchanges and the energy efficiency of household ponds in the dike-pond system of the Zhujiang Delta, China. Bull Natl Museum Ethnol 11:323–343
Rugani B, Panasiuk D, Benetto E (2012) An input–output based framework to evaluate human labour in life cycle assessment. Int J Life Cycle Assess 17:795–812. doi:10.1007/s11367-012-0403-1
Samuel-Fitwi B, Meyer S, Reckmann K et al (2013) Aspiring for environmentally conscious aquafeed: comparative LCA of aquafeed manufacturing using different protein sources. J Clean Prod 52:225–233
Schroeder GL (1978) Autotrophic and heterotrophic production of micro-organisms in intensely-manured fish ponds, and related fish yields. Aquaculture 14:303–325
Schroeder GL (1987a) Carbon and nitrogen budgets in manured fish ponds on Israel’s coastal plain. Aquaculture 62:259–279
Schroeder GL (1987b) carbon pathways in aquatic detrital systems. In: Moriarty DJW, Pullin RSV (eds) Detritus Microb. Ecol. Aquac. ICLARM, Manila, pp 217–236
Selvam BP, Natchimuthu S, Bastviken D (2014) Methane and carbon dioxide emissions from inland waters in India - implications for large scale greenhouse gas balances Department of Thematic Studies – Water and Environmental Studies, Linköping University, Current address: Department of Physical Geography
Tucker CS, Hargreaves JA, Boyd CE (2008) Better management practices for freshwater pond aquaculture. In: Tucker CS, Hargreaves JA (eds) Environ. best Manag. Pract. Aquac. Wiley-Blackwell, Oxford, pp 151–226
Turner PA, Griffis TJ, Lee X et al (2015) Indirect nitrous oxide emissions from streams within the US Corn Belt scale with stream order. Proc Natl Acad Sci. doi:10.1073/pnas.1503598112
Van der Walt S, Colbert SC, Varoquaux G (2011) The NumPy array: a structure for efficient numerical computation. Comput Sci Eng 13:22–30
Weidema BP, Bauer C, Hischier R et al (2013) Overview and methodology. Data quality guideline for the ecoinvent database version 3. Ecoinvent Rep1(v3):St
Williams J, Crutzen PJ (2010) Nitrous oxide from aquaculture. Nat Geosci 3:143
Williams AG, Audsley E, Sandars DL (2010) Environmental burdens of producing bread wheat, oilseed rape and potatoes in England and Wales using simulation and system modelling. Int J Life Cycle Assess 15:855–868
Wong Chor Yee A (1999) New developments in integrated dike-pond agriculture-aquaculture in the Zhujiang delta, China: Ecological implications. Ambio 28:529–533
Xiao S, Yang H, Liu D et al (2014) Gas transfer velocities of methane and carbon dioxide in a subtropical shallow pond. Tellus B 66:1–14
Xiaohua W, Zhenmin F (2001) Rural household energy consumption with the economic development in China: stages and characteristic indices. Energy Policy 29:1391–1397
Yang N (2005a) Cultured aquatic species information programme. Hypophthalmichthys molitrix. In: FAO Fish Aquac Dep. http://www.fao.org/fishery/culturedspecies/Hypophthalmichthys_nobilis/en#tcNA00EA. Accessed 15 Jul 2015
Yang N (2005b) Cultured aquatic species information programme. Hypophthalmichthys nobilis. In: FAO Fish Aquac Dep. http://www.fao.org/fishery/culturedspecies/Hypophthalmichthys_nobilis/en#tcNA00EA. Accessed 15 Jul 2015
Zhong G (1982) The mulberry dike-fish pond complex: a Chinese ecosystem of land-water interaction on the Pearl River Delta. Hum Ecol 10:191–202
Zhu Y, Yang Y, Wan J et al (1990) The effect of manure application rate and frequency upon fish yield in integrated fish farm ponds. Aquaculture 91:233–251
Acknowledgments
We thank the European Research Council (SP2-GA-2008-233409 and PoC 324607) for funding and the anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ian Vázquez-Rowe
Electronic supplementary material
Yields, emission factors, production parameters and uncertainty estimates are provided in the Electronic Supplementary Material.
ESM 1
(PDF 528 kb)
Rights and permissions
About this article
Cite this article
Astudillo, M.F., Thalwitz, G. & Vollrath, F. Modern analysis of an ancient integrated farming arrangement: life cycle assessment of a mulberry dyke and pond system. Int J Life Cycle Assess 20, 1387–1398 (2015). https://doi.org/10.1007/s11367-015-0950-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-015-0950-3