Abstract
Aging is associated with the onset and progression of multiple diseases, which limit health span. Chronic low-grade inflammation in the absence of overt infection is considered the simmering source that triggers age-associated diseases. Failure of many cellular processes during aging is mechanistically linked to inflammation; however, the overall decline in the cellular homeostasis mechanism of autophagy has emerged as one of the top and significant inducers of inflammation during aging, frequently known as inflammaging. Thus, physiological or pharmacological interventions aimed at improving autophagy are considered geroprotective. Rapamycin analogs (rapalogs) are known for their ability to inhibit mTOR and thus regulate autophagy. This study assessed the efficacy of everolimus, a rapalog, in regulating inflammatory cytokine production in T cells from older adults. CD4+ T cells from older adults were treated with a physiological dose of everolimus (0.01 µM), and indices of autophagy and inflammation were assessed to gain a mechanistic understanding of the effect of everolimus on inflammation. Everolimus (Ever) upregulated autophagy and broadly alleviated inflammatory cytokines produced by multiple T cell subsets. Everolimus’s ability to alleviate the cytokines produced by Th17 subsets of T cells, such as IL-17A and IL-17F, was dependent on autophagy and antioxidant signaling pathways. Repurposing the antineoplastic drug everolimus for curbing inflammaging is promising, given the drug’s ability to restore multiple cellular homeostasis mechanisms.
Graphical Abstract
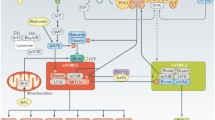
Everolimus inhibits mTORC1 and promotes autophagy in CD4 + T cells from O adults, which induces the activation and translocation of NRF2 to the nucleus. NRF2 induces the production of first-line antioxidant enzymes SOD1, SOD2, and catalase, thus lowering reactive oxygen species and proinflammatory cytokine production







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Single-cell RNA sequencing data is available on Gene Expression Omnibus (GEO), accession number GSE 241492. Graphical abstract was created using Biorender.
References
Saxton RA, Sabatini DM. MTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–76.
Mannick JB, Lamming DW. Targeting the biology of aging with MTOR inhibitors. Nat Aging. 2023;3:642–60.
Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, et al. MTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6(268):ra179. https://doi.org/10.1126/scitranslmed.3009892.
Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12.
Wang Y, Li YB, Yin JJ, Wang Y, Zhu LB, Xie GY, Pan SH. Autophagy regulates inflammation following oxidative injury in diabetes. Autophagy. 2013;9:272–7.
Zhu Q, Wang R, Nemecio D, Liang C. How autophagy is tied to inflammation and cancer. Mol Cell Oncol 2020;7 https://doi.org/10.1080/23723556.2020.1717908.
Fujikake N, Shin M, Shimizu S. Association between autophagy and neurodegenerative diseases. Front Neurosci 2018;12.
Wu DJ, Adamopoulos IE. Autophagy and autoimmunity. Clin Immunol. 2017;176:55–62.
Bharath LP, Rockhold JD, Conway R. Selective autophagy in hyperglycemia‐induced microvascular and macrovascular diseases. Cells 2021;10.
Goldberg EL, Dixit VD. Drivers of age-related inflammation and strategies for healthspan extension. Immunol Rev. 2015;265:63–74.
Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54:437–53.
Conway R, Rockhold JD, Santacruz-calvo S, Zukowski E, Pugh GH, Hasturk H, Kern PA, Nikolajczyk BS, Bharath, LP. Obesity and fatty acids promote mitochondrial translocation of STAT3 through ROS-dependent mechanisms. 2022;3:1–11. https://doi.org/10.3389/fragi.2022.924003.
Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, Jiang K, Liu R, Guo Z, Deeney J, et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 2020;32:44-55.e6. https://doi.org/10.1016/j.cmet.2020.04.015.
Valente AJ, Maddalena LA, Robb EL, Moradi F, Stuart JA. A Simple ImageJ macro tool for analyzing mitochondrial network morphology in mammalian cell culture. Acta Histochem. 2017;119:315–26. https://doi.org/10.1016/j.acthis.2017.03.001.
Kirber MT, Chen K, Keany JF. YFP photoconversion revisited: confirmation of the CFP-like species. Nat Methods. 2007;4:767–8.
Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–32. https://doi.org/10.1111/j.1365-2818.2006.01706.x.
Adler J, Parmryd I. Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytometry A. 2010;77:733–42. https://doi.org/10.1002/cyto.a.20896.
Ip B, Cilfone NA, Belkina AC, Defuria J, Jagannathan-Bogdan M, Zhu M, Kuchibhatla R, McDonnell ME, Xiao Q, Kepler TB, et al. Th17 cytokines dif-ferentiate obesity from obesity-associated type 2 diabetes and promote TNFα production. Obesity. 2016;24:102–12. https://doi.org/10.1002/oby.21243.
Zukowski E, Sannella M, Rockhold JD, Kalantar GH, Yu J, SantaCruz‐Calvo S, Kuhn MK, Hah N, Ouyang L, Wang T. et al. STAT3 modulates CD4+ T mitochondrial dynamics and function in aging. Aging Cell 2023;22. https://doi.org/10.1111/acel.13996.
Zheng GXY, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, Ziraldo SB, Wheeler TD, McDermott GP, Zhu J. et al. Massively parallel digital tran-scriptional profiling of single cells. Nat Commun 2017;8.https://doi.org/10.1038/ncomms14049.
Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573-3587.e29. https://doi.org/10.1016/j.cell.2021.04.048.
Bais AS, Kostka D. Scds: Computational annotation of doublets in single-cell RNA sequencing data. Bioinformatics. 2020;36:1150–8. https://doi.org/10.1093/bioinformatics/btz698.
Wold S, Ruhe A, Wold H, Dunn IWJ. The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. SIAM J Sci Stat Comput. 1984;5:735–43. https://doi.org/10.1137/0905052.
Geladi P, Kowalski BP. Partial least-squares regression: a tutorial. Anal Chim Acta. 1986;185:1–17.
Thévenot EA, Roux A, Xu Y, Ezan E, Junot C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical. J Proteome Res. 2015;14:3322–35.
Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS). J Chemom. 2002;16:119–28.
Galindo-Prieto B, Eriksson L, Trygg J. Variable influence on projection (VIP) for orthogonal projections to latent structures (OPLS). J Chemom. 2014;28:623–32.
Lee JS, Lee WW, Kim SH, Kang Y, Lee N, Shin MS, Kang SW, Kang I. Age-associated alteration in naive and memory Th17 cell response in humans. Clin Immunol. 2011;140:84–91. https://doi.org/10.1016/j.clim.2011.03.018.
Barbosa MC, Grosso RA, Fader CM. Hallmarks of aging: an autophagic perspective. Front Endocrinol (Lausanne) 2019;9. https://doi.org/10.3389/fendo.2018.00790.
Chen J, Liu X, Zhong Y. Interleukin-17A: the key cytokine in neurodegenerative diseases. Front Aging Neurosci 2020;12. https://doi.org/10.3389/fnagi.2020.566922.
Mohammadi Shahrokhi V, Ravari A, Mirzaei T, Zare-Bidaki M, Asadikaram G, Arababadi MK. IL-17A and IL-23: plausible risk factors to induce age-associated in-flammation in Alzheimer’s disease. Immunol Invest. 2018;47:812–22. https://doi.org/10.1080/08820139.2018.1504300.
Tan J, Dai A, Pan L, Zhang L, Wang Z, Ke T, Sun W, Wu Y, Ding P-H, Chen L. Inflamm-aging-related cytokines of IL-17 and IFN-γ accelerate osteoclasto-genesis and periodontal destruction. J Immunol Res. 2021;2021:1–12. https://doi.org/10.1155/2021/9919024.
Chen W, Wang J, Yang H, Sun Y, Chen B, Liu Y, Han Y, Shan M, Zhan J. Interleukin 22 and its association with neurodegenerative disease activity. Front Pharmacol 2022;13. https://doi.org/10.3389/fphar.2022.958022.
Chen X, Wang Y, Wang J, Wen J, Jia X, Wang X, Zhang H. Accumulation of T helper 22 cells, interleukin 22 and myeloid derived suppressor cells promotes gastric cancer progression in elderly patients. Oncol Lett. 2018. https://doi.org/10.3892/ol.2018.8612.
Pollizzi KN, Powell JD. Regulation of T cells by MTOR: the known knowns and the known unknowns. Trends Immunol. 2015;36:13–20. https://doi.org/10.1016/j.it.2014.11.005.
Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The MTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–44. https://doi.org/10.1016/j.immuni.2009.04.014.
Yang K, Shrestha S, Zeng H, Karmaus PWF, Neale G, Vogel P, Guertin DA, Lamb RF, Chi H. T cell exit from quiescence and differentiation into Th2 cells depend on raptor-MTORC1-mediated metabolic reprogramming. Immunity. 2013;39:1043–56. https://doi.org/10.1016/j.immuni.2013.09.015.
Merkley SD, Chock CJ, Yang XO, Harris J, Castillo EF. Modulating T cell responses via autophagy: the intrinsic influence controlling the function of both anti-gen-presenting cells and T cells. Front Immunol 2018;9. https://doi.org/10.3389/fimmu.2018.02914.
Bektas A, Schurman SH, Gonzalez-Freire M, Dunn CA, Singh AK, Macian F, Cuervo AM, Sen R, Ferrucci L. Age-associated changes in human CD4+ T cells point to mitochondrial dysfunction consequent to impaired autophagy. Aging. 2019;11:9234–63. https://doi.org/10.18632/aging.102438.
Belikov AV, Schraven B, Simeoni L. T cells and reactive oxygen species. J Biomed Sci. 2015;22:85. https://doi.org/10.1186/s12929-015-0194-3.
Redza-Dutordoir M, Averill-Bates DA. Interactions between reactive oxygen species and autophagy. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2021;1868:119041. https://doi.org/10.1016/j.bbamcr.2021.119041.
Zhao Z, Wang Y, Gao Y, Ju Y, Zhao Y, Wu Z, Gao S, Zhang B, Pang X, Zhang Y. et al. The PRAK-NRF2 axis promotes the differentiation of Th17 cells by mediating the redox homeostasis and glycolysis. Proc Natl Acad Sci 2023;120. https://doi.org/10.1073/pnas.2212613120.
Yang X, Gao X, Zhu Y, Li G, Zhang X, Xiao G, Cao H, Chen X, Yang Q, Lu B. ATF4 is induced by ROS and regulates Th1 and Th17 cells (P6219). J Immunol. 2013;190:115.5-115.5. https://doi.org/10.4049/jimmunol.190.Supp.115.5.
Yarosz EL, Chang C-H. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw 2018;18. https://doi.org/10.4110/in.2018.18.e14.
Reinke EN, Ekoue DN, Bera S, Mahmud N, Diamond AM. Translational regulation of GPx-1 and GPx-4 by the MTOR pathway. PLoS One. 2014;9:e93472. https://doi.org/10.1371/journal.pone.0093472.
Yun HR, Jo YH, Kim J, Shin Y, Kim SS, Choi TG. Roles of autophagy in oxidative stress. Int J Mol Sci. 2020;21:3289. https://doi.org/10.3390/ijms21093289.
Chang K-C, Liu P-F, Chang C-H, Lin Y-C, Chen Y-J, Shu C-W. The interplay of autophagy and oxidative stress in the pathogenesis and therapy of retinal degenerative diseases. Cell Biosci. 2022;12:1. https://doi.org/10.1186/s13578-021-00736-9.
Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–88. https://doi.org/10.1038/cdd.2014.150.
Schmidlin CJ, Dodson MB, Madhavan L, Zhang DD. Redox regulation by NRF2 in aging and disease. Free Radic Biol Med. 2019;134:702–7. https://doi.org/10.1016/j.freeradbiomed.2019.01.016.
Alsaleh G, Panse I, Swadling L, Zhang H, Richter FC, Meyer A, Lord J, Barnes E, Klenerman P, Green C. et al. Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. Elife 2020;9. https://doi.org/10.7554/eLife.57950.
Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I, Palikaras K, Simonsen A, Johansen T, Tavernarakis N, et al. Autophagy in healthy aging and disease. Nat Aging. 2021;1:634–50. https://doi.org/10.1038/s43587-021-00098-4.
Katsuragi Y, Ichimura Y, Komatsu M. Regulation of the Keap1–Nrf2 pathway by P62/SQSTM1. Curr Opin Toxicol. 2016;1:54–61. https://doi.org/10.1016/j.cotox.2016.09.005.
Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou Y-S, Ueno I, Sakamoto A, Tong KI, et al. The selective autophagy substrate P62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–23. https://doi.org/10.1038/ncb2021.
Kageyama S, Saito T, Obata M, Koide R, Ichimura Y, Komatsu M. Negative regulation of the Keap1-Nrf2 pathway by a P62/Sqstm1 splicing variant. Mol Cell Biol 2018;38. https://doi.org/10.1128/MCB.00642-17.
Tsang CK, Chen M, Cheng X, Qi Y, Chen Y, Das I, Li X, Vallat B, Fu L-W, Qian C-N, et al. SOD1 phosphorylation by MTORC1 couples nutrient sensing and redox regulation. Mol Cell. 2018;70:502-515.e8. https://doi.org/10.1016/j.molcel.2018.03.029.
Kong H, Chandel NS. To claim growth Turf, MTOR says SOD off. Mol Cell. 2018;70:383–4. https://doi.org/10.1016/j.molcel.2018.04.015.
Zhang Y, Swanda RV, Nie L, Liu X, Wang C, Lee H, Lei G, Mao C, Koppula P, Cheng W, et al. MTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat Commun. 2021;12:1589. https://doi.org/10.1038/s41467-021-21841-w.
Chávez MD, Tse HM. Targeting mitochondrial-derived reactive oxygen species in T cell-mediated autoimmune diseases. Front Immunol 2021;12. https://doi.org/10.3389/fimmu.2021.703972.
Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, AnandhBabu PV, et al. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can J Physiol Pharmacol. 2014;92:605–12. https://doi.org/10.1139/cjpp-2014-0017.
Bharath LP, Cho JM, Park S-K, Ruan T, Li Y, Mueller R, Bean T, Reese V, Richardson RS, Cai J, et al. Endothelial cell autophagy maintains shear stress–induced nitric oxide generation via glycolysis-dependent purinergic signaling to endo-thelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2017;37:1646–56. https://doi.org/10.1161/ATVBAHA.117.309510.
Vereb G, Matkó J, Vámosi G, Ibrahim SM, Magyar E, Varga S, Szöllösi J, Jenei A, Gáspár R, Waldmann TA, et al. Cholesterol-dependent clustering of IL-2Rα and its colocalization with HLA and CD48 on T lymphoma cells suggest their functional association with lipid rafts. Proc Natl Acad Sci U S A. 2000;97:6013–8. https://doi.org/10.1073/pnas.97.11.6013.
Acknowledgements
We would like to thank Thuy Trang (Violet) T. Tran, Lab Manager, Department of Biology and Chemistry and the Departments of Biology and Chemistry, Merrimack College. We also thank Tara Daly, Research Program Manager, Center for Health Inclusion Research and Practice(CHIRP), Merrimack College.
Funding
This work was supported by R15AG068957 (LPB), pilot award from the San Diego Nathan Shock Center of Excellence in the Basic Biology of Aging P30AG068635 (LPB), R56AG06985 (BSN), and T32NS115667 (MKK). This work was also supported by the Pasini Fellowship (LPB), College of Health Sciences Faculty Development grant (FDG) (LPB), and Sakowich Center for Undergraduate Research and Creative Activities grant (SCURCA), School of Nursing and Health Sciences, Merrimack College (LPB), and Barnstable Brown Diabetes Center, University of Kentucky (BSN).
Author information
Authors and Affiliations
Contributions
Conceptualization: LPB. Methodology: LPB. Investigation: JDR, HM, MS, KG, LM, EZ, GK, SSC, SD, OS, GC, and LPB. Formal analysis: JDR, HM, MS, JY, EP, MKK, EAP, KG, LM, OS, and GC. Supervision: LPB. Writing: LPB. Editing: BSN, HH. Funding: LPB, BSN. Resources: HH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Rockhold, J.D., Marszalkowski, H., Sannella, M. et al. Everolimus alleviates CD4+ T cell inflammation by regulating autophagy and cellular redox homeostasis. GeroScience (2024). https://doi.org/10.1007/s11357-024-01187-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01187-z