Abstract
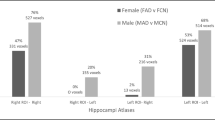
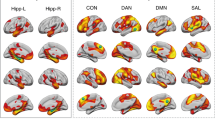
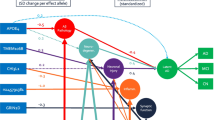
As of 2023, it is estimated that 6.7 million individuals in the United States live with Alzheimer’s disease (AD). Prior research indicates that AD disproportionality affects females; females have a greater incidence rate, perform worse on a variety of neuropsychological tasks, and have greater total brain atrophy. Recent research shows that hippocampal functional connectivity differs by sex and may be related to the observed sex differences in AD, and apolipoprotein E (ApoE) ε4 carriers have reduced hippocampal functional connectivity. The purpose of this study was to determine if the ApoE genotype plays a role in the observed sex differences in hippocampal functional connectivity in Alzheimer’s disease. The resting state fMRI and T2 MRI of individuals with AD (n = 30, female = 15) and cognitively normal individuals (n = 30, female = 15) from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) were analyzed using the functional connectivity toolbox (CONN). Our results demonstrated intrahippocampal functional connectivity differed between those without an ε4 allele and those with at least one ε4 allele in each group. Additionally, intrahippocampal functional connectivity differed only by sex when Alzheimer’s participants had at least one ε4 allele. These results improve our current understanding of the role of the interacting relationship between sex, ApoE genotype, and hippocampal function in AD. Understanding these biomarkers may aid in the development of sex-specific interventions for improved AD treatment.




Similar content being viewed by others
Data availability
All data derived from the ADNI and that specific to this study are available to researchers by request as outlined in the ADNI access policy (adni.loni.usc.edu).
References
Alzheimer's Association. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023;19(4):1598–95.
National Institute of Health. Alzheimer’s disease. 2021. Available from https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/alzheimers-disease. Accessed 30 Jan 2023.
Alzheimer's Association. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700–89.
National Institute on Aging. What happens to the brain in Alzheimer’s disease? National Institute on Aging; 2017.
Guo T, et al. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener. 2020;15(1):1–37.
Chapman RM, et al. Women have farther to fall: gender differences between normal elderly and Alzheimer’s disease in verbal memory engender better detection of Alzheimer’s disease in women. J Int Neuropsychol Soc. 2011;17(4):654–62.
Gumus M, et al. Progression of neuropsychiatric symptoms in young-onset versus late-onset Alzheimer’s disease. Geroscience. 2021;43:213–23.
Henderson VW, Buckwalter JG. Cognitive deficits of men and women with Alzheimer’s disease. Neurology. 1994;44(1):90–6.
Andrew MK, Tierney MC. The puzzle of sex, gender and Alzheimer’s disease: why are women more often affected than men? Womens Health. 2018;14:1745506518817995.
Mielke MM. Sex and gender differences in Alzheimer’s disease dementia. Psychiatr Times. 2018;35(11):14–7.
Mielke MM, et al. Consideration of sex and gender in Alzheimer’s disease and related disorders from a global perspective. Alzheimers Dement. 2022;18(12):2707–24.
Pearce EE, et al. Telomere length and epigenetic clocks as markers of cellular aging: a comparative study. Geroscience. 2022;44(3):1861–9.
Guerreiro R, Bras J. The age factor in Alzheimer’s disease. Genome Med. 2015;7(1):1–3.
Li Z, et al. APOE2: protective mechanism and therapeutic implications for Alzheimer’s disease. Mol Neurodegener. 2020;15(1):63.
Sando SB, et al. APOE ε4 lowers age at onset and is a high risk factor for Alzheimer’s disease; a case control study from central Norway. BMC Neurol. 2008;8:1–7.
Yamazaki Y, et al. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol. 2019;15(9):501–18.
Michaelson DM. APOE ε4: the most prevalent yet understudied risk factor for Alzheimer’s disease. Alzheimers Dement. 2014;10(6):861–8.
Liu C-C, et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–18.
Emrani S, et al. APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: a systematic review. Alzheimers Res Ther. 2020;12(1):141.
Sienski G, et al. APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci Transl Med. 2021;13(583):eaaz4564.
Canuet L, et al. Resting-state network disruption and APOE genotype in Alzheimer’s disease: a lagged functional connectivity study. PLoS ONE. 2012;7(9): e46289.
Jelic V, et al. Apolipoprotein E ε4 allele decreases functional connectivity in Alzheimer’s disease as measured by EEG coherence. J Neurol Neurosurg Psychiatry. 1997;63(1):59–65.
Adluru N, et al. Sex differences of APOE neuropathology-based scores in brain aging. Alzheimers Dement. 2023;19: e064003.
Neu SC, et al. Apolipoprotein E genotype and sex risk factors for alzheimer disease: a meta-analysis. JAMA Neurol. 2017;74(10):1178–89.
Sundermann EE, et al. Sex differences in the association between apolipoprotein E ε4 allele and Alzheimer’s disease markers. Alzheimers Dementia Diagn Assess Dis Monit. 2018;10:438–47.
Burke SL, et al. Sex differences in the development of mild cognitive impairment and probable Alzheimer’s disease as predicted by hippocampal volume or white matter hyperintensities. J Women Aging. 2019;31(2):140–64.
Ardekani BA, Convit A, Bachman AH. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. J Alzheimers Dis. 2016;50:847–57.
Deutschendorf L. Gender differences in the functional connectivity of the hippocampus in Alzheimer’s disease. 2023, Dissertation, Tilburg University.
Williamson J, et al. Sex difference in brain functional connectivity of hippocampus in Alzheimer’s disease. GeroScience. 2024;46(1):563–72.
Williamson J, et al. Sex differences in brain functional connectivity of hippocampus in mild cognitive impairment. Frontiers in Aging Neuroscience. 2022;14: 959394.
Cavedo E, et al. Sex differences in functional and molecular neuroimaging biomarkers of Alzheimer’s disease in cognitively normal older adults with subjective memory complaints. Alzheimers Dement. 2018;14(9):1204–15.
Heise V, et al. Apolipoprotein E genotype, gender and age modulate connectivity of the hippocampus in healthy adults. Neuroimage. 2014;98:23–30.
Weiner MW. Alzheimer’s disease neuroimaging initiative. Available from https://adni.loni.usc.edu/. Accessed 30 Jan 2023.
Whitfield-Gabriel S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–41.
Jenkinson M, et al. Fsl. Neuroimage. 2012;62(2):782–90.
Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19.
Woolrich MW, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl):S173–86.
Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89.
Nieto-Castanon A. Handbook of functional connectivity magnetic resonance imaging methods in CONN. Boston, MA: Hilbert Press; 2020.
Worsley KJ, et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4(1):58–73.
Chen Y, et al. Disrupted functional and structural networks in cognitively normal elderly subjects with the APOE ɛ4 allele. Neuropsychopharmacology. 2015;40(5):1181–91.
Fleisher AS, et al. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuroimage. 2009;47(4):1678–90.
Matura S, et al. Recognition memory is associated with altered resting-state functional connectivity in people at genetic risk for Alzheimer’s disease. Eur J Neurosci. 2014;40(7):3128–35.
Turney IC, et al. APOE ε4 and resting-state functional connectivity in racially/ethnically diverse older adults. Alzheimers Dementia Diagn Assess Dis Monit. 2020;12(1): e12094.
Zerbi V, et al. Resting-state functional connectivity changes in aging apoE4 and apoE-KO mice. J Neurosci. 2014;34(42):13963–75.
Shen J, et al. Modulation of APOE and SORL1 genes on hippocampal functional connectivity in healthy young adults. Brain Struct Funct. 2017;222(6):2877–89.
Wang X, et al. Apolipoprotein E ε4 modulates cognitive profiles, hippocampal volume, and resting-state functional connectivity in Alzheimer’s disease. J Alzheimers Dis. 2015;45:781–95.
Manno FA, et al. Early stage alterations in white matter and decreased functional interhemispheric hippocampal connectivity in the 3xTg mouse model of Alzheimer’s disease. Front Aging Neurosci. 2019;11:39.
Wang Z, et al. Interhemispheric functional and structural disconnection in Alzheimer’s disease: a combined resting-state fMRI and DTI study. PLoS ONE. 2015;10(5): e0126310.
Aganj I, et al. Compensatory brain connection discovery in Alzheimer’s disease. Proc IEEE Int Symp Biomed Imaging. 2020;2020:283–7.
Gardini S, et al. Increased functional connectivity in the default mode network in mild cognitive impairment: a maladaptive compensatory mechanism associated with poor semantic memory performance. J Alzheimers Dis. 2015;45:457–70.
Liu Y, et al. APOE genotype and neuroimaging markers of Alzheimer’s disease: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86(2):127–34.
Lin H, et al. Sex modulates the apolipoprotein E ε4 effect on white matter and cortical functional connectivity in individuals with amnestic mild cognitive impairment. Eur J Neurol. 2020;27(8):1415–21.
Hohman TJ, et al. Sex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75(8):989–98.
Nyarko JNK, et al. Profiles of β-amyloid peptides and key secretases in brain autopsy samples differ with sex and APOE ε4 status: impact for risk and progression of Alzheimer disease. Neuroscience. 2018;373:20–36.
Harrison TM, et al. Tau deposition is associated with functional isolation of the hippocampus in aging. Nat Commun. 2019;10(1):4900.
Rehbein E, et al. Shaping of the female human brain by sex hormones: a review. Neuroendocrinology. 2021;111(3):183–206.
Dose J, et al. APOE genotype and stress response - a mini review. Lipids Health Dis. 2016;15(1):121.
Yin J, et al. Effect of ApoE isoforms on mitochondria in Alzheimer disease. Neurology. 2020;94(23):e2404–11.
Robinson JL, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141(7):2181–93.
Gamache J, Yun Y, Chiba-Falek O. Sex-dependent effect of APOE on Alzheimer’s disease and other age-related neurodegenerative disorders. Dis Model Mech. 2020;13(8):211.
Casula EP, et al. Regional precuneus cortical hyperexcitability in Alzheimer’s disease patients. Ann Neurol. 2023;93(2):371–83.
Koch G, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169:302–11.
Acknowledgements
Alzheimer’s disease neuroimaging initiative investigators:
Michael Weiner9, Paul Aisen10, Ronald Petersen11, Clifford R. Jack Jr11, William Jagust12, John Q. Trojanowki13, Arthur W. Toga14, Laurel Beckett15, Robert C. Green16, Andrew J. Saykin17, John C. Morris27, Leslie M. Shaw13, Enchi Liu18, Tom Montine19, Ronald G. Thomas10, Michael Donohue10, Sarah Walter10, Devon Gessert10, Tamie Sather10, Gus Jiminez10, Danielle Harvey15, Matthew Bernstein10, Nick Fox20, Paul Thompson21, Norbert Schuff22, Charles DeCArli15, Bret Borowski11, Jeff Gunter11, Matt Senjem11, Prashanthi Vemuri11, David Jones11, Kejal Kantarci11, Chad Ward11, Robert A. Koeppe23, Norm Foster24, Eric M. Reiman25, Kewei Chen25, Chet Mathis26, Susan Landau12, Nigel J. Cairns27, Erin Householder14, Lisa Taylor Reinwald27, Virginia Lee28, Magdalena Korecka28, Michal Figurski28, Karen Crawford14, Scott Neu14, Tatiana M. Foroud17, Steven Potkin29, Li Shen17, Faber Kelley17, Sungeun Kim17, Kwangsik Nho17, Zaven Kachaturian30, Richard Frank31, Peter J. Snyder32, Susan Molchan33, Jeffrey Kaye34, Joseph Quinn34, Betty Lind34, Raina Carter34, Sara Dolen34, Lon S. Schneider14, Sonia Pawluczyk14, Mauricio Beccera14, Liberty Teodoro14, Bryan M. Spann14, James Brewer10, Helen Vanderswag10, Adam Fleisher10, Judith L. Heidebrink23, Joanne L. Lord23, Ronald Petersen11, Sara S. Mason11, Colleen S. Albers11, David Knopman11, Kris Johnson11, Rachelle S. Doody35, Javier Villanueva Meyer35, Munir Chowdhury35, Susan Rountree35, Mimi Dang35, Yaakov Stern36, Lawrence S. Honig36, Karen L. Bell36, Beau Ances27, Maria Carroll27, Sue Leon27, Erin Householder27, Mark A. Mintun27, Stacy Schneider27, Angela OliverNG37, Randall Griffith37, David Clark37, David Geldmacher37, John Brockington37, Erik Roberson37, Hillel Grossman38, Effie Mitsis38, Leyla deToledo-Morrell39, Raj C. Shah39, Ranjan Duara40, Daniel Varon40, Maria T. Greig40, Peggy Roberts40, Marilyn Albert41, Chiadi Onyike41, Daniel D’Agostino II41, Stephanie Kielb41, James E. Galvin42, Dana M. Pogorele42, Brittany Cerbone42, Christina A. Michel42, Henry Rusinek42, Mony J. de Leon42, Lidia Glodzik42, Susan De Santi42, P. Murali Doraiswamy43, Jeffrey R. Petrella43, Terence Z. Wong43, Steven E. Arnold13, Jason H. Karlawish13, David A. Wolk26, Charles D. Smith44, Greg Jicha44, Peter Hardy44, Partha Sinha44, Elizabeth Oates44, Gary Conrad44, Oscar L. Lopez26, MaryAnn Oakley26, Donna M. Simpson26, Anton P. Porsteinsson45, Bonnie S. Goldstein45, Kim Martin45, Kelly M. Makino45, M. Saleem Ismail45, Connie Brand45, Ruth A. Mulnard29, Gaby Thai29, Catherine Mc Adams Ortiz29, Kyle Womack46, Dana Mathews46, Mary Quiceno46, Ramon Diaz Arrastia46, Richard King46, Myron Weiner46, Kristen Martin Cook46, Michael DeVous46, Allan I. Levey47, James J. Lah47, Janet S. Cellar47, Jeffrey M. Burns48, Heather S. Anderson48, Russell H. Swerdlow48, Liana Apostolova49, Kathleen Tingus49, Ellen Woo49, Daniel H. S. Silverman49, Po H. Lu49, George Bartzokis49, Neill R. Graff Radford50, Francine Parfitt50, Tracy Kendall50, Heather Johnson50, Martin R. Farlow17, Ann Marie Hake17, Brandy R. Matthews17, Scott Herring17, Cynthia Hunt17, Christopher H. van Dyck51, Richard E. Carson51, Martha G. MacAvoy51, Howard Chertkow52, Howard Bergman52, Chris Hosein52, Sandra Black53, Bojana Stefanovic53, Curtis Caldwell53, Ging Yuek Robin Hsiung54, Howard Feldman54, Benita Mudge54, Michele Assaly Past54, Andrew Kertesz55, John Rogers55, Dick Trost55, Charles Bernick56, Donna Munic56, Diana Kerwin57, Marek Marsel Mesulam57, Kristine Lipowski57, Chuang Kuo Wu57, Nancy Johnson57, Carl Sadowsky58, Walter Martinez58, Teresa Villena58, Raymond Scott Turner59, Kathleen Johnson59, Brigid Reynolds59, Reisa A. Sperling16, Keith A. Johnson16, Gad Marshall16, Meghan Frey16, Jerome Yesavage60, Joy L. Taylor60, Barton Lane60, Allyson Rosen60, Jared Tinklenberg60, Marwan N. Sabbagh61, Christine M. Belden61, Sandra A. Jacobson61, Sherye A. Sirrel61, Neil Kowall62, Ronald Killiany62, Andrew E. Budson62, Alexander Norbash62, Patricia Lynn Johnson62, Thomas O. Obisesan63, Saba Wolday63, Joanne Allard63, Alan Lerner64, Paula Ogrocki64, Leon Hudson64, Evan Fletcher65, Owen Carmichael65, John Olichney65, Charles DeCarli65, Smita Kittur66, Michael Borrie67, T. Y. Lee67, Rob Bartha67, Sterling Johnson68, Sanjay Asthana68, Cynthia M. Carlsson68, Steven G. Potkin69, Adrian Preda69, Dana Nguyen69, Pierre Tariot25, Adam Fleisher25, Stephanie Reeder25, Vernice Bates70, Horacio Capote70, Michelle Rainka70, Douglas W. Scharre71, Maria Kataki71, Anahita Adeli71, Earl A. Zimmerman72, Dzintra Celmins72, Alice D. Brown72, Godfrey D. Pearlson73, Karen Blank73, Karen Anderson73, Robert B. Santulli74, Tamar J. Kitzmiller74, Eben S. Schwartz74, Kaycee M. Sink75, Jeff D. Williamson75, Pradeep Garg75, Franklin Watkins75, Brian R. Ott76, Henry Querfurth76, Geoffrey Tremont76, Stephen Salloway77, Paul Malloy77, Stephen Correia77, Howard J. Rosen9, Bruce L. Miller9, Jacobo Mintzer78, Kenneth Spicer78, David Bachman78, Elizabether Finger79, Stephen Pasternak79, Irina Rachinsky79, John Rogers79, Andrew Kertesz79, Dick Drost79, Nunzio Pomara80, Raymundo Hernando80, Antero Sarrael80, Susan K. Schultz81, Laura L. Boles Ponto81, Hyungsub Shim81, Karen Elizabeth Smith81, Norman Relkin82, Gloria Chaing82, Lisa Raudin82, Amanda Smith83, Kristin Fargher83 & Balebail Ashok Raj83
9UC San Francisco, San Francisco, CA, USA. 10University of California San Diego, San Diego, CA, USA. 11Mayo Clinic, Rochester, NY, USA. 12UC Berkeley, Berkeley, CA, USA. 13University of Pennsylvania, Philadelphia, PA, USA. 14University of Southern California, Los Angeles, CA, USA. 15Davis, Davis, CA, USA. 16Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA. 17Indiana University, Bloomington, IND, USA. 18Janssen Alzheimer Immunotherapy, South San Francisco, CA, USA. 19University of Washington, Seattle, WA, USA. 20University of London, London, UK. 21USC School of Medicine, Los Angeles, CA, USA. 22UCSF MRI, San Francisco, CA, USA. 23University of Michigan, Ann Arbor, MI, USA. 24University of Utah, Salt Lake City, UT, USA. 25Banner Alzheimer’s Institute, Phoenix, AZ, USA. 26University of Pittsburgh, Pittsburgh, PA, USA. 27Washington University St. Louis, St. Louis, MO, USA. 28UPenn School of Medicine, Philadelphia, PA, USA. 29University of California, Irvine, CA, USA. 30Khachaturian, Radebaugh & Associates, Inc and Alzheimer’s Association’s Ronald and Nancy Reagan’s Research Institute, Chicago, IL, USA. 31General Electric, Boston, MA, USA. 32Brown University, Providence, RI, USA. 33National Institute on Aging/National Institutes of Health, Bethesda, MD, USA. 34Oregon Health and Science University, Portland, OR, USA. 35Baylor College of Medicine, Houston, TX, USA. 36Columbia University Medical Center, New York, NY, USA. 37University of Alabama Birmingham, Birmingham, MO, USA. 38Mount Sinai School of Medicine, New York, NY, USA. 39Rush University Medical Center, Chicago, IL, USA. 40Wien Center, Vienna, Austria. 41Johns Hopkins University, Baltimore, MD, USA. 42New York University, New York, NY, USA. 43Duke University Medical Center, Durham, NC, USA. 44University of Kentucky, Lexington, KY, USA. 45University of Rochester Medical Center, Rochester, NY, USA. 46University of Texas Southwestern Medical School, Dallas, TX, USA. 47Emory University, Atlanta, GA, USA. 48University of Kansas, Medical Center, Lawrence, KS, USA. 49University of California, Los Angeles, CA USA. 50Mayo Clinic, Jacksonville, FL, USA. 51Yale University School of Medicine, New Haven, CT, USA. 52McGill Univ., Montreal Jewish General Hospital, Montreal, WI, USA. 53Sunnybrook Health Sciences, Toronto, ON, Canada. 54U.B.C. Clinic for AD & Related Disorders, British Columbia, BC, Canada. 55Cognitive Neurology St. Joseph’s, Toronto, ON, Canada. 56Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV, USA. 57Northwestern University, Evanston, IL, USA. 58Premiere Research Inst Palm Beach Neurology, West Palm Beach, FL, USA. 59Georgetown University Medical Center, Washington, DC, USA. 60Stanford University, Santa Clara County, CA, USA. 61Banner Sun Health Research Institute, Sun City, AZ, USA. 62Boston University, Boston, MA, USA. 63Howard University, Washington, DC, USA. 64Case Western Reserve University, Cleveland, OH, USA. 65University of California, Davis Sacramento, CA, USA. 66Neurological Care of CNY, New York, NY, USA. 67Parkwood Hospital, Parkwood, CA, USA. 68University of Wisconsin, Madison, WI, USA. 69University of California, Irvine BIC, Irvine, CA, USA. 70Dent Neurologic Institute, Amherst, MA, USA. 71Ohio State University, Columbus, OH, USA. 72Albany Medical College, Albany, NY, USA. 73Hartford Hosp, Olin Neuropsychiatry Research Center, Hartford, CT, USA. 74Dartmouth Hitchcock Medical Center, Albany, NY, USA. 75Wake Forest University Health Sciences, Winston-Salem, NC, USA. 76Rhode Island Hospital, Providence, RI, USA. 77Butler Hospital, Providence, RI, USA. 78Medical University South Carolina, Charleston, SC, USA. 79St. Joseph’s Health Care, Toronto, ON, Canada. 80Nathan Kline Institute, Orangeburg, SC, USA. 81University of Iowa College of Medicine, Iowa City, IA, USA. 82Cornell University, Ithaca, NY, USA. 83University of South Florida: USF Health Byrd Alzheimer’s Institute, Tampa, FL, USA.
Funding
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. This work was also partially supported by the American Heart Association (AHA834339) and the National Institute on Aging of National Institutes of Health (R01AG075834) awards. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Consortia
Contributions
J.W. conducted the study and drafted the manuscript. Y.Y. contributed to conceptualization, problem-solving, and guidance during the conduction of the study. S.J., S.M., B.S., T.W., P.M, A.Y., and Y.Y. participated in editing the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Boards of all participating ADNI sites reviewed and approved the data collection protocols provided by ADNI.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf and in the “Acknowledgements” section.
About this article
Cite this article
Williamson, J.N., James, S.A., Mullen, S.P. et al. Sex differences in interacting genetic and functional connectivity biomarkers in Alzheimer’s disease. GeroScience (2024). https://doi.org/10.1007/s11357-024-01151-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-024-01151-x