Abstract
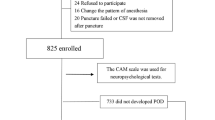
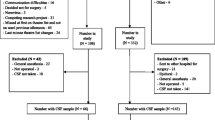
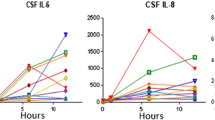
Postoperative delirium (POD) is a common neuropsychiatric complication in geriatric inpatients after hip fracture surgery and its occurrence is associated with poor outcomes. The purpose of this study was to investigate the relationship between preoperative biomarkers in serum and cerebrospinal fluid (CSF) and the development of POD in older hip fracture patients, exploring the possibility of integrating objective methods into future predictive models of delirium. Sixty hip fracture patients were recruited. Blood and CSF samples were collected at the time of spinal anesthesia when none of the subjects had delirium. Patients were assessed daily using the 4AT scale, and based on these results, they were divided into POD and non-POD groups. The Olink® platform was used to analyze 45 cytokines. Twenty-one patients (35%) developed POD. In the subsample of 30 patients on whom proteomic analyses were performed, a proteomic profile was associated with the incidence of POD. Chemokine (C-X-C motif) ligand 9 (CXCL9) had the strongest correlation between serum and CSF samples in patients with POD (rho = 0.663; p < 0.05). Although several cytokines in serum and CSF were associated with POD after hip fracture surgery in older adults, there was a significant association with lower preoperative levels of CXCL9 in CSF and serum. Despite the small sample size, this study provides preliminary evidence of the potential role of molecular biomarkers in POD, which may provide a basis for the development of new delirium predictive models.




Similar content being viewed by others
References
Inouye SK, Westendorp RGJ, Saczynski JS, Naeije G, Pepersack T. Delirium in elderly people. Lancet. 2014;383(9920):911–22. https://doi.org/10.1016/S0140-6736(13)60688-1.
Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456–66. https://doi.org/10.1056/nejmcp1605501.
Bruce AJ, Ritchie CW, Blizard R, Lai R, Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatrics. 2007;19(2):197–214. https://doi.org/10.1017/S104161020600425X.
Zhou J, Liu C, Sun Y, Huang W, Ye K. Cognitive disorders associated with hospitalization of COVID-19: results from an observational cohort study. Brain Behav Immun. 2021;91:383–92. https://doi.org/10.1016/j.bbi.2020.10.019.
Oberai T, Woodman R, Laver K, Crotty M, Kerkhoffs G, Jaarsma R. Is delirium associated with negative outcomes in older patients with hip fracture: analysis of the 4904 patients 2017–2018 from the Australian and New Zealand hip fracture registry. ANZ J Surg. 2022;92(1–2):200–5. https://doi.org/10.1111/ans.17421.
Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190–222. https://doi.org/10.1016/j.jagp.2013.09.005.
Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, MacLullich AMJ, Slooter AJC, Ely EW. Delirium. Nat Rev Dis Prim. 2020;6(1). https://doi.org/10.1038/s41572-020-00223-4.
Wang Y, Shen X. Postoperative delirium in the elderly: the potential neuropathogenesis. Aging Clin Exp Res. 2018;30(11):1287–95. https://doi.org/10.1007/s40520-018-1008-8.
Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–56. https://doi.org/10.1016/j.ebiom.2018.10.021.
Subramaniyan S, Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg. 2019;128(4):781–8. https://doi.org/10.1213/ANE.0000000000004053.
Liu X, Yu Y, Zhu S. Inflammatory markers in postoperativedelirium (POD) and cognitive dysfunction (POCD): A meta-analysis of observational studies. PLoS One. 2018;13(4). https://doi.org/10.1371/journal.pone.0195659.
Beloosesky Y, Hendel D, Weiss A, Hershkovitz A, Grinblat J, Pirotsky A, et al. Cytokines and C-reactive protein production in hip-fracture-operated elderly patients. Journals Gerontol - Ser A Biol Sci Med Sci. 2007;62(4):420–6. https://doi.org/10.1093/gerona/62.4.420.
Hirsch J, Vacas S, Terrando N, Yuan M, Sands LP, Kramer J, et al. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation. 2016;13(1):211. https://doi.org/10.1186/s12974-016-0681-9.
Miao S, Shen P, Zhang Q, Wang H, Shen J, Wang G, et al. Neopterin and mini-mental state examination scores, two independent risk factors for postoperative delirium in elderly patients with open abdominal surgery. J Cancer Res Ther. 2018;14(6):1234–8. https://doi.org/10.4103/0973-1482.192764.
Freter S, Dunbar M, Koller K, MacKnight C, Rockwood K. Risk of pre- and post-operative delirium and the delirium elderly at risk (DEAR) tool in hip fracture patients. Can Geriatr J. 2015;18(4):212–6. https://doi.org/10.5770/cgj.18.185.
Zhao S, Sun T, Zhang J, Chen X, Wang X. Risk factors and prognosis of postoperative delirium in nonagenarians with hip fracture. Sci Rep. 2023;13(1):1–7. https://doi.org/10.1038/s41598-023-27829-4.
Smith TO, Cooper A, Peryer G, Griffiths R, Fox C, Cross J. Factors predicting incidence of post-operative delirium in older people following hip fracture surgery: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2017;32(4):386–96. https://doi.org/10.1002/gps.4655.
Auer S, Reisberg B. The GDS/FAST staging system. Int Psychogeriatrics. 1997;9(SUPPL. 1):167–71. https://doi.org/10.1017/S1041610297004869.
Barthel M. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:56–61.
Lawton M, Brody E. Assessment of older people: selfmaintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86.
Woo J, Yu R, Wong M, Yeung F, Wong M, Lum C. Frailty screening in the community using the FRAIL scale. J Am Med Dir Assoc. 2015;16(5):412–9. https://doi.org/10.1016/j.jamda.2015.01.087.
Kaiser MJ, Bauer JM, Ramsh C, Uter W, Guigoz Y, Cederlorm T, et al. Validation of the mini nutritional assessment short-form (MNA®-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782–8https://doi.org/10.1007/s12603-009-0214-7.
Herdman M, Badia X, Berra S. EuroQol-5D: a simple alternative for measuring health-related quality of life in primary care. Aten Primaria. 2001;28(6):425–30. https://doi.org/10.1016/s0212-6567(01)70406-4.
Yesavage JA. Geriatric depression scale. Psychopharmacol Bull. 1988;24(4):709–10. https://doi.org/10.1007/978-3-319-69892-2_736-1.
Tieges Z, Maclullich AMJ, Anand A, Brookes C, Cassarino M, O’connor M, et al. Diagnostic accuracy of the 4AT for delirium detection in older adults: systematic review and meta-analysis. Age Ageing. 2021;50(3):733–43. https://doi.org/10.1093/ageing/afaa224.
Delgado-Parada E, Morillo-Cuadrado D, Saiz-Ruiz J, Cebollada-Gracia A, Ayuso-Mateos JL, Cruz-Jentoft AJ. Diagnostic accuracy of the Spanish version of the 4AT scale (4AT-ES) for delirium screening in older inpatients. Eur J Psychiatry. 2022;36(3):182–90. https://doi.org/10.1016/j.ejpsy.2022.01.003.
Barahona E, Pinhao R, Galindo V, Noguera A. The diagnostic sensitivity of the memorial delirium assessment scale—Spanish version. J Pain Symptom Manage. 2018;55(3):968–72. https://doi.org/10.1016/j.jpainsymman.2017.11.013.
Meagher D, Adamis D, Leonard M, Trzepacz P, Grover S, Jabbar F, et al. Development of an abbreviated version of the delirium motor subtyping scale (DMSS-4). Int Psychogeriatrics. 2014;26(4):693–702. https://doi.org/10.1017/S1041610213002585.
Burton JK, Stott DJ, McShane R, Noel-Storr AH, Swann-Price RS, Quinn TJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the early detection of dementia across a variety of healthcare settings. Cochrane Database Syst Rev. 2021;7(7). https://doi.org/10.1002/14651858.CD011333.pub3.
Blandfort S, Gregersen M, Rahbek K, Juul S, Damsgaard EM. The short IQCODE as a predictor for delirium in hospitalized geriatric patients. Aging Clin Exp Res. 2020;32(10):1969–76. https://doi.org/10.1007/s40520-019-01412-2.
Luo J, Frisken S, Machado I, Zhang M, Pieper S, Golland P, et al. Using the variogram for vector outlier screening: application to feature-based image registration. Int J Comput Assist Radiol Surg. 2018;13(12):1871–80. https://doi.org/10.1007/s11548-018-1840-5.
Metsalu T, Vilo J. ClustVis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43(W1):W566–70. https://doi.org/10.1093/nar/gkv468.
Torres KCL, de Rezende VB, Lima-Silva ML, de Sousa SLJ, Costa CG, de Melo MJV, et al. Immune senescence and biomarkers profile of Bambuí aged population-based cohort. Exp Gerontol. 2018;103:47–56. https://doi.org/10.1016/j.exger.2017.12.006.
Sayed N, Huang Y, Nguyen K, Krejciova-Rajaniemi Z, Grawe AP, Gao T, et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat Aging. 2021;1(8):748. https://doi.org/10.1038/s43587-021-00102-x.
Phan QT, Chua KY, Jin A, Winkler C, Koh WP. CXCL9 predicts the risk of osteoporotic hip fracture in a prospective cohort of Chinese men—a matched case–control study. J Bone Miner Res. 2022;37(10):1843–9. https://doi.org/10.1002/jbmr.4646.
de Amorim JSC, Torres KCL, Teixeira-Carvalho A, Martins-Filho OA, Lima-Costa MF, Peixoto SV. Inflammatory markers and occurrence of falls: Bambuí cohort study of aging. Rev Saude Publica. 2019;53:1–11. https://doi.org/10.11606/S1518-8787.2019053000855.
de Amorim JSC, Torres KCL, Carvalho AT, Martins-Filho OA, Lima-Costa MF, Peixoto SV. Inflammatory markers associated with fall recurrence and severity: the Bambuí cohort study of aging. Exp Gerontol. 2020;132: 110837. https://doi.org/10.1016/j.exger.2020.110837.
Watson AES, Goodkey K, Footz T, Voronova A. Regulation of CNS precursor function by neuronal chemokines. Neurosci Lett. 2020;715: 134533. https://doi.org/10.1016/j.neulet.2019.134533.
Satarkar D, Patra C. Evolution, expression and functional analysis of CXCR3 in neuronal and cardiovascular diseases: a narrative review. Front Cell Dev Biol. 2022;10(June):1–17. https://doi.org/10.3389/fcell.2022.882017.
Lee KS, Chung JH, Lee KH, Shin MJ, Oh BH, Hong CH. Bioplex analysis of plasma cytokines in Alzheimer’s disease and mild cognitive impairment. Immunol Lett. 2008;121(2):105–9. https://doi.org/10.1016/j.imlet.2008.09.004.
Koper OM, Kaminska J, Sawicki K, Kemona H. CXCL9, CXCL10, CXCL11, and their receptor (CXCR3) in neuroinflammation and neurodegeneration. Adv Clin Exp Med. 2018;27(6):849–56. https://doi.org/10.17219/acem/68846.
Soares HD, Potter WZ, Pickering E, Kuhn M, Immermann FW, Shera DM, et al. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch Neurol. 2012;69(10):1310–7. https://doi.org/10.1001/archneurol.2012.1070.
Berchiche YA, Sakmar TP. CXC chemokine receptor 3 alternative splice variants selectively activate different signaling pathways. Mol Pharmacol. 2016;90(4):483–95. https://doi.org/10.1124/mol.116.105502.
Li H, Wang R. A focus on CXCR4 in Alzheimer’s disease. Brain Circ. 2017;3(4):199. https://doi.org/10.4103/bc.bc_13_17.
Pellecchia MT, Santangelo G, Picillo M, Pivonello R, Longo K, Pivonello C, et al. Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson’s disease patients. J Neurol. 2013;260(2):438–44. https://doi.org/10.1007/s00415-012-6648-6.
Yasar Z, Elliott BT, Kyriakidou Y, Nwokoma CT, Postlethwaite RD, Gaffney CJ, et al. Sprint interval training (SIT) reduces serum epidermal growth factor (EGF), but not other inflammatory cytokines in trained older men. Eur J Appl Physiol. 2021;121(7):1909–19. https://doi.org/10.1007/s00421-021-04635-2.
Sanchez-Ramos J, Song S, Sava V. Granulocyte colony stimulating factor (G-CSF) decreases brain amyloid burden and reverses cognitive impairment in Alzheimer’s mice. Neuroscience. 2009;163(1):55–72. https://doi.org/10.1016/j.neuroscience.2009.05.071.
Tuo M, Xiao Y, Xu Y, Wang L, Wei X, Zhang L. Role of granulocyte-colony stimulating factor in the protection of cerebral vascular endothelium, white matter, and cognition. Curr Neurovasc Res. 2019;16(5):425–32. https://doi.org/10.2174/1567202616666191029115113.
Khedr EM, Gomaa AMS, Ahmed OG, Sayed HMM, Gamea A. Cognitive impairment, P300, and transforming growth factor β1 in different forms of dementia. J Alzheimer’s Dis. 2020;78(2):837–45. https://doi.org/10.3233/JAD-200885.
Estrada LD, Oliveira-Cruz L, Cabrera D. Transforming growth factor beta type I role in neurodegeneration: implications for Alzheimer´s disease. Curr Protein Pept Sci. 2018;19(12):1180–8. https://doi.org/10.2174/1389203719666171129094937.
Khan SH, Lindroth H, Jawed Y, Wang S, Nasser J, Seyffert S, et al. Serum biomarkers in postoperative delirium after esophagectomy. Ann Thorac Surg. 2022;113(3):1000–7. https://doi.org/10.1016/j.athoracsur.2021.03.035.
Culjak M, Perkovic MN, Uzun S, Strac DS, Erjavec GN, Leko MB, et al. The association between TNF-alpha, IL-1 alpha and IL-10 with Alzheimer’s disease. Curr Alzheimer Res. 2020;17(11):972–84. https://doi.org/10.2174/1567205017666201130092427.
Ahmad MA, Kareem O, Khushtar M, Akbar M, Haque MR, Iqubal A, et al. Neuroinflammation: a potential risk for dementia. Int J Mol Sci. 2022;23(2):616. https://doi.org/10.3390/ijms23020616.
Lee WJ, Liao YC, Wang YF, Lin IF, Wang SJ, Fuh JL. Plasma MCP-1 and cognitive decline in patients with Alzheimer’s disease and mild cognitive impairment: a two-year follow-up study. Sci Rep. 2018;8(1):4–11. https://doi.org/10.1038/s41598-018-19807-y.
Smith RJ, Lachner C, Singh VP, Trivedi S, Khatua B, Cartin-Ceba R. Cytokine profiles in intensive care unit delirium. Acute Crit Care. 2022;37(3):415–28. https://doi.org/10.4266/acc.2021.01508.
Perna L, Trares K, Perneczky R, Tato M, Stocker H, Möllers T, et al. Risk of late-onset depression and cognitive decline: results from inflammatory proteome analyses in a prospective population-based cohort study. Am J Geriatr Psychiatry. 2022;30(6):689–700. https://doi.org/10.1016/j.jagp.2021.12.001.
Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease - associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013;33:183–9. https://doi.org/10.1016/j.bbi.2013.07.007.
Dogrul RT, Dogrul AB, Konan A, Caglar O, Sumer F, Caliskan H, et al. Does preoperative comprehensive geriatric assessment and frailty predict postoperative complications? World J Surg. 2020;44(11):3729–36. https://doi.org/10.1007/s00268-020-05715-8.
Yang Y, Zhao X, Gao L, Wang Y, Wang J. Incidence and associated factors of delirium after orthopedic surgery in elderly patients: a systematic review and meta-analysis. Aging Clin Exp Res. 2021;33(6):1493–506. https://doi.org/10.1007/s40520-020-01674-1.
Acknowledgements
We would like to thank the patients and staff at the Orthopedics and Anesthesiology Departments of Hospital Universitario de Navarra. We also thank the Clinical Neuroproteomics Unit of Navarrabiomed and the Navarrabiomed Biobank for supporting this study. We thank RRV, AGJ, and RRO for their assistance in statistics and data interpretation. The sponsors had no role in the design and conduct of the study, the collection, management, analysis, or interpretation of the data.
Funding
This work was supported by a grant from the Department of Economic and Business Development from the Government of Navarra (Ref. 0011–1411-2020–000028).
Author information
Authors and Affiliations
Contributions
LLV, NMV, JROGand BACV designed the study. RRV and AGJ performed statistical analyses and interpreted data. LLV acquired interpreted data and drafted the manuscript. MMV, RRV, MI, FZ, BACV, BVM, JROG, AMHO, AJMV, JFI, RRO, and ESM critically revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Lozano-Vicario, L., Muñoz-Vázquez, Á.J., Ramírez-Vélez, R. et al. Association of postoperative delirium with serum and cerebrospinal fluid proteomic profiles: a prospective cohort study in older hip fracture patients. GeroScience 46, 3235–3247 (2024). https://doi.org/10.1007/s11357-024-01071-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-024-01071-w