Abstract
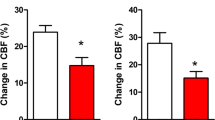
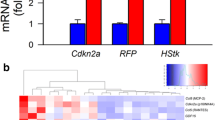
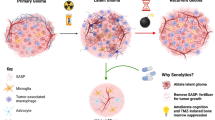
Whole brain irradiation (WBI), a commonly employed therapy for multiple brain metastases and as a prophylactic measure after cerebral metastasis resection, is associated with a progressive decline in neurocognitive function, significantly impacting the quality of life for approximately half of the surviving patients. Recent preclinical investigations have shed light on the multifaceted cerebrovascular injury mechanisms underlying this side effect of WBI. In this study, we aimed to test the hypothesis that WBI induces endothelial senescence, contributing to chronic disruption of the blood-brain barrier (BBB) and microvascular rarefaction. To accomplish this, we utilized transgenic p16-3MR mice, which enable the identification and selective elimination of senescent cells. These mice were subjected to a clinically relevant fractionated WBI protocol (5 Gy twice weekly for 4 weeks), and cranial windows were applied to both WBI-treated and control mice. Quantitative assessment of BBB permeability and capillary density was performed using two-photon microscopy at the 6-month post-irradiation time point. The presence of senescent microvascular endothelial cells was assessed by imaging flow cytometry, immunolabeling, and single-cell RNA-sequencing (scRNA-seq). WBI induced endothelial senescence, which associated with chronic BBB disruption and a trend for decreased microvascular density in the mouse cortex. In order to investigate the cause-and-effect relationship between WBI-induced senescence and microvascular injury, senescent cells were selectively removed from animals subjected to WBI treatment using Navitoclax/ABT263, a well-known senolytic drug. This intervention was carried out at the 3-month post-WBI time point. In WBI-treated mice, Navitoclax/ABT263 effectively eliminated senescent endothelial cells, which was associated with decreased BBB permeability and a trend for increased cortical capillarization. Our findings provide additional preclinical evidence that senolytic treatment approaches may be developed for prevention of the side effects of WBI.




Similar content being viewed by others
References
Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54.
Jung EW, Rakowski JT, Delly F, Jagannathan J, Konski AA, Guthikonda M, Kim H, Mittal S. Gamma knife radiosurgery in the management of brainstem metastases. Clin Neurol Neurosurg. 2013;115:2023–8.
Welzel G, Fleckenstein K, Schaefer J, Hermann B, Kraus-Tiefenbacher U, Mai SK, Wenz F. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys. 2008;72:1311–8.
Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70.
Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–42.
Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818–22.
Johannesen TB, Lien HH, Hole KH, Lote K. Radiological clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother Oncol. 2003;69:169–76.
Welzel G, Fleckenstein K, Mai SK, Hermann B, Kraus-Tiefenbacher U, Wenz F. Acute neurocognitive impairment during cranial radiation therapy in patients with intracranial tumors. Strahlenther Onkol. 2008;184:647–54.
Silber JH, Radcliffe J, Peckham V, Perilongo G, Kishnani P, Fridman M, Goldwein JW, Meadows AT. Whole-brain irradiation decline in intelligence: the influence of dose age on IQ score. J Clin Oncol. 1992;10:1390–6.
Lamproglou I, Martin S, Diserbo M, Multon E, Petiet A, Colas-Linhart N, Bok B, Martin C. Total body 4.5 Gy gamma irradiation-induced early delayed learning memory dysfunction in the rat. Cell Mol Biol (Noisy-le-grand). 2001;47:453–7.
Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D'Agostino R, Brunso-Bechtold JK. Spatial learning memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166:892–9.
Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy chemotherapy. Lancet. 2009;374:1639–51.
Warrington JP, Csiszar A, Mitschelen M, Lee YW, Sonntag WE. Whole brain radiation-induced impairments in learning memory are time-sensitive reversible by systemic hypoxia. PLoS One. 2012;7:e30444.
Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fulop GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42.
Ungvari Z, Podlutsky A, Sosnowska D, Tucsek Z, Toth P, Deak F, Gautam T, Csiszar A, Sonntag WE. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage decreased DNA repair capacity in microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci. 2013;68:1443–57.
Yabluchanskiy A, Tarantini S, Balasubramanian P, Kiss T, Csipo T, Fulop GA, Lipecz A, Ahire C, DelFavero J, Nyul-Toth A, Sonntag WE, Schwartzman ML, Campisi J, Csiszar A, Ungvari Z. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. Geroscience. 2020;42:409–28.
Warrington JP, Ashpole N, Csiszar A, Lee YW, Ungvari Z, Sonntag WE. Whole brain radiation-induced vascular cognitive impairment: mechanisms implications. J Vasc Res. 2013;50:445–57.
Ashpole NM, Warrington JP, Mitschelen MC, Yan H, Sosnowska D, Gautam T, Farley JA, Csiszar A, Ungvari Z, Sonntag WE. Systemic influences contribute to prolonged microvascular rarefaction after brain irradiation: a role for endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2014;307:H858–68.
Warrington JP, Csiszar A, Johnson DA, Herman TS, Ahmad S, Lee YW, Sonntag WE. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am J Physiol Heart Circ Physiol. 2011;300:H736–44.
Lo EH, Frankel KA, Steinberg GK, DeLaPaz RL, Fabrikant JI. High-dose single-fraction brain irradiation: MRI, cerebral blood flow, electrophysiological, histological studies. Int J Radiat Oncol Biol Phys. 1992;22:47–55.
Wilhelm I, Nyul-Toth A, Kozma M, Farkas AE, Krizbai IA. Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am J Physiol Heart Circ Physiol. 2017;313:H1000–12.
Costea L, Meszaros A, Bauer H, Bauer HC, Traweger A, Wilhelm I, Farkas AE, Krizbai IA. The blood-brain barrier its intercellular junctions in age-related brain disorders. Int J Mol Sci. 2019;20(21):E5472. https://doi.org/10.3390/ijms20215472.
Bezek S, Trnovec T, Scasnar V, Durisova M, Kukan M, Kallay Z, Laginova V, Svoboda V. Irradiation of the head by 60Co opens the blood-brain barrier for drugs in rats. Experientia. 1990;46:1017–20.
Fauquette W, Amourette C, Dehouck MP, Diserbo M. Radiation-induced blood-brain barrier damages: an in vitro study. Brain Res. 2012;1433:114–26.
Yoshida Y, Sejimo Y, Kurachi M, Ishizaki Y, Nakano T, Takahashi A. X-ray irradiation induces disruption of the blood-brain barrier with localized changes in claudin-5 activation of microglia in the mouse brain. Neurochem Int. 2018;119:199–206.
Yuan H, Gaber MW, Boyd K, Wilson CM, Kiani MF, Merchant TE. Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, ultrastructural changes. Int J Radiat Oncol Biol Phys. 2006;66:860–6.
Yuan H, Gaber MW, McColgan T, Naimark MD, Kiani MF, Merchant TE. Radiation-induced permeability leukocyte adhesion in the rat blood-brain barrier: modulation with anti-ICAM-1 antibodies. Brain Res. 2003;969:59–69.
Allen BD, Limoli CL. Breaking barriers: Neurodegenerative repercussions of radiotherapy induced damage on the blood-brain blood-tumor barrier. Free Radic Biol Med. 2022;178:189–201.
Moretti R, Caruso P. An iatrogenic model of brain small-vessel disease: post-radiation encephalopathy. Int J Mol Sci. 2020;21(18):6506. https://doi.org/10.3390/ijms21186506.
Nair V, Roth LJ. Effect of X-irradiation certain other treatments on blood brain barrier permeability. Radiat Res. 1964;23:249–64.
Remler MP, Marcussen WH, Tiller-Borsich J. The late effects of radiation on the blood brain barrier. Int J Radiat Oncol Biol Phys. 1986;12:1965–9.
Nation DA, Sweeney MD, Montagne A, Sagare AP, D'Orazio LM, Pachicano M, Sepehrb F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW, Zlokovic BV. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–6.
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease back. Physiol Rev. 2019;99:21–78.
Coppe JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J. Tumor suppressor aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem. 2011;286:36396–403.
Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–9.
Tarantini S, Balasubramanian P, Delfavero J, Csipo T, Yabluchanskiy A, Kiss T, Nyul-Toth A, Mukli P, Toth P, Ahire C, Ungvari A, Benyo Z, Csiszar A, Ungvari Z. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience. 2021;43:2427–40.
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–9.
Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis creates a pro-regenerative environment. Nat Med. 2017;23:775–81.
Abdul-Aziz AM, Sun Y, Hellmich C, Marlein CR, Mistry J, Forde E, Piddock RE, Shafat MS, Morfakis A, Mehta T, Di Palma F, Macaulay I, Ingham CJ, Haestier A, Collins A, Campisi J, Bowles KM, Rushworth SA. Acute myeloid leukemia induces protumoral p16INK4a-driven senescence in the bone marrow microenvironment. Blood. 2019;133:446–56.
Kim HN, Chang J, Iyer S, Han L, Campisi J, Manolagas SC, Zhou D, Almeida M. Elimination of senescent osteoclast progenitors has no effect on the age-associated loss of bone mass in mice. Aging Cell. 2019;18:e12923.
Patil P, Dong Q, Wang D, Chang J, Wiley C, Demaria M, Lee J, Kang J, Niedernhofer LJ, Robbins PD, Sowa G, Campisi J, Zhou D, Vo N. Systemic clearance of p16(INK4a) -positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell. 2019;18:e12927.
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkl JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–6.
Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkl JL, Khosla S. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23:1072–9.
Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkl JL. Targeting senescent cells enhances adipogenesis metabolic function in old age. Elife. 2015;4:e12997. https://doi.org/10.7554/eLife.12997.
Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkl JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–7.
Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IWF, Houtsmuller AB, Pothof J, de Bruin RWF, Madl T, Hoeijmakers JHJ, Campisi J de Keizer PLJ. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity aging. Cell. 2017;169(132-147):e16.
Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–33.
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83.
Demaria M, O'Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM, Alston S, Academia EC, Kilmarx S, Valdovinos A, Wang B, de Bruin A, Kennedy BK, Melov S, Zhou D, et al. Cellular senescence promotes adverse effects of chemotherapy cancer relapse. Cancer Discov. 2017;7:165–76.
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkl JL. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–35.
Li B, Yabluchanskiy A, Tarantini S, Allu SR, Sencan-Egilmez I, Leng J, Alfadhel MAH, Porter JE, Fu B, Ran C, Erdener SE, Boas DA, Vinogradov SA, Sonntag WE, Csiszar A, Ungvari Z, Sakadzic S. Measurements of cerebral microvascular blood flow, oxygenation, morphology in a mouse model of whole-brain irradiation-induced cognitive impairment by two-photon microscopy optical coherence tomography: evidence for microvascular injury in the cerebral white matter. Geroscience. 2023;45(3):1491–510. https://doi.org/10.1007/s11357-023-00735-3.
Institoris A, Murphy-Royal C, Tarantini S, Yabluchanskiy A, Haidey JN, Csiszar A, Ungvari Z, Gordon GR. Whole brain irradiation in mice causes long-term impairment in astrocytic calcium signaling but preserves astrocyte-astrocyte coupling. Geroscience. 2021;43:197–212.
Nyul-Toth A, Tarantini S, DelFavero J, Yan F, Balasubramanian P, Yabluchanskiy A, Ahire C, Kiss T, Csipo T, Lipecz A, Farkas AE, Wilhelm I, Krizbai IA, Tang Q, Csiszar A, Ungvari Z. Demonstration of age-related blood-brain barrier disruption cerebromicrovascular rarefaction in mice by longitudinal intravital two-photon microscopy optical coherence tomography. Am J Physiol Heart Circ Physiol. 2021;320:H1370–92.
Ahire C, Nyul-Toth A, DelFavero J, Gulej R, Faakye JA, Tarantini S, Kiss T, Kuan-Celarier A, Balasubramanian P, Ungvari A, Tarantini A, Nagaraja R, Yan F, Tang Q, Mukli P, Csipo T, Yabluchanskiy A, Campisi J, Ungvari Z, Csiszar A. Accelerated cerebromicrovascular senescence contributes to cognitive decline in a mouse model of paclitaxel (Taxol)-induced chemobrain. Aging Cell. 2023;22(7):e13832. https://doi.org/10.1111/acel.13832.
Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, Satija R. Comprehensive Integration of Single-Cell Data. Cell. 2019;177(1888-1902):e21.
Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, species. Nat Biotechnol. 2018;36:411–20.
Ilicic T, Kim JK, Kolodziejczyk AA, Bagger FO, McCarthy DJ, Marioni JC, Teichmann SA. Classification of low quality cells from single-cell RNA-seq data. Genome Biol. 2016;17:29.
Luecken MD, Theis FJ. Current best practices in single-cell RNA-seq analysis: a tutorial. Mol Syst Biol. 2019;15:e8746.
Hafemeister C, Satija R. Normalization variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20:296.
Ahlmann-Eltze C, Huber W. glmGamPoi: fitting Gamma-Poisson generalized linear models on single cell count data. Bioinformatics. 2021;36:5701–2.
Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech: Theory Exp. 2008;2008:P10008.
Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, DelFavero J, Yabluchanskiy A, Csipo T, Farkas E, Wiley G, Garman L, Csiszar A, Ungvari Z. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience. 2020;42:429–44.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50.
Carnero A. Markers of cellular senescence. Methods Mol Biol. 2013;965:63–81.
Nagano T, Nakano M, Nakashima A, Onishi K, Yamao S, Enari M, Kikkawa U, Kamada S. Identification of cellular senescence-specific genes by comparative transcriptomics. Sci Rep. 2016;6:31758.
Van de Sande B, Flerin C, Davie K, De Waegeneer M, Hulselmans G, Aibar S, Seurinck R, Saelens W, Cannoodt R, Rouchon Q, Verbeiren T, De Maeyer D, Reumers J, Saeys Y, Aerts S. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat Protoc. 2020;15:2247–76.
Kalucka J, de Rooij L, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, Garcia-Caballero M, Khan S, Geldhof V, Sokol L, Chen R, Treps L, Borri M, de Zeeuw P, Dubois C, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180(764-779):e20.
Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, Ungvari Z, Csiszar A. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience. 2021;43:2387–94.
Tarantini S, Balasubramanian P, Yabluchanskiy A, Ashpole NM, Logan S, Kiss T, Ungvari A, Nyul-Toth A, Schwartzman ML, Benyo Z, Sonntag WE, Csiszar A, Ungvari Z. IGF1R signaling regulates astrocyte-mediated neurovascular coupling in mice: implications for brain aging. Geroscience. 2021;43:901–11.
Brown WR, Blair RM, Moody DM, Thore CR, Ahmed S, Robbins ME, Wheeler KT. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia. J Neurol Sci. 2007;257:67–71.
Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164:662–8.
Elfarnawany M, Pinter SZ, Lacefield JC. Improved objective selection of power Doppler wall-filter cut-off velocity for accurate vascular quantification. Ultrasound Med Biol. 2012;38:1429–39.
Turnquist C, Beck JA, Horikawa I, Obiorah IE, Von Muhlinen N, Vojtesek B, Lane DP, Grunseich C, Chahine JJ, Ames HM, Smart DD, Harris BT, Harris CC. Radiation-induced astrocyte senescence is rescued by Delta133p53. Neuro-Oncology. 2019;21:474–85.
Casella G, Munk R, Kim KM, Piao Y, De S, Abdelmohsen K, Gorospe M. Transcriptome signature of cellular senescence. Nucleic Acids Res. 2019;47:7294–305.
Yamazaki Y, Baker DJ, Tachibana M, Liu CC, van Deursen JM, Brott TG, Bu G, Kanekiyo T. Vascular cell senescence contributes to blood-brain barrier breakdown. Stroke. 2016;47:1068–77.
Lee WH, Cho HJ, Sonntag WE, Lee YW. Radiation attenuates physiological angiogenesis by differential expression of VEGF, Ang-1, tie-2 Ang-2 in rat brain. Radiat Res. 2011;176:753–60.
Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function neurovascular coupling responses improves cognitive function in aged mice. Redox Biol. 2019;24:101192.
Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309:H1837–45.
Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787.
Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302.
Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–50.
Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21:1318–31.
Kerkhofs D, Wong SM, Zhang E, Uiterwijk R, Hoff EI, Jansen JFA, Staals J, Backes WH, van Oostenbrugge RJ. Blood-brain barrier leakage at baseline cognitive decline in cerebral small vessel disease: a 2-year follow-up study. Geroscience. 2021;43:1643–52.
Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, response to cocoa in older people. Neurology. 2013;81:904–9.
Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell'Oglio E, Meier DS, Newton E, Milberg WP, Guttmann CR, Lipsitz LA. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol. 2011;70:213–20.
Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–81.
Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation Alzheimer's disease J Gerontol A Biol Sci Med Sci 2014;69:1212-1226.
Parente A, de Vries EFJ, van Waarde A, Ioannou M, van Luijk P, Langendijk JA, Dierckx R, Doorduin J. The acute early effects of whole-brain irradiation on glial activation, brain metabolism, behavior: a positron emission tomography study. Mol Imaging Biol. 2020;22:1012–20.
Dorigatti AO, Riordan R, Yu Z, Ross G, Wang R, Reynolds-Lallement N, Magnusson K, Galvan V, Perez VI. Brain cellular senescence in mouse models of Alzheimer's disease. Geroscience. 2022;44:1157–68.
Kiss T, Nyul-Toth A, DelFavero J, Balasubramanian P, Tarantini S, Faakye J, Gulej R, Ahire C, Ungvari A, Yabluchanskiy A, Wiley G, Garman L, Ungvari Z, Csiszar A. Spatial transcriptomic analysis reveals inflammatory foci defined by senescent cells in the white matter, hippocampi cortical grey matter in the aged mouse brain. Geroscience. 2022;44:661–81.
Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15:555–65.
Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–21.
Acknowledgements
This work was supported by grants from the American Heart Association (ANT: AHA834339) and (ST: AHA CDA941290), the Oklahoma Center for the Advancement of Science and Technology, the National Institute on Aging (RF1AG072295, R01AG055395, R01AG068295; R01AG070915, K01AG073614, K01AG073613, R03AG070479), the National Institute of Neurological Disorders and Stroke (R01NS100782), the National Cancer Institute (R01CA255840), the Oklahoma Shared Clinical and Translational Resources (U54GM104938) with an Institutional Development Award (IDeA) from NIGMS, the Presbyterian Health Foundation, the Reynolds Foundation, the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (P20GM125528), the NCI Cancer Center Support Grant (P30 CA225520), and the Oklahoma Tobacco Settlement Endowment Trust. Analysis help was provided by the Oklahoma Medical Research Foundation Quantitative Analysis Core supported by COBRE grant 1 P30 GM110766-01 and the Oklahoma Shared Clinical and Translational Resources (U54GM104938) with an Institutional Development Award (IDeA) from NIGMS. PP was supported by the Intramural Program of NIAAA/NIH. TK was supported by Project no. TKP2021-NKTA-47, implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme; by funding through the National Cardiovascular Laboratory Program (RRF-2.3.1-21-2022-00003) provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund; Project no. 135784 implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K_20 funding scheme and the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/ EUniWell/EAC-A02-2019/EAC-A02-2019-1). The authors express their gratitude for Unity Biotechnology, Inc. for providing the p16-3MR mouse model. The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the American Heart Association, or the Presbyterian Health Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Anna Csiszar and Dr. Pal Pacher serve as Associate Editor for The Journal of Gerontology, Series A: Biological Sciences and Medical Sciences and GeroScience. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience and as Consulting Editor for The American Journal of Physiology-Heart and Circulatory Physiology. Dr. Pal Pacher, Dr. Stefano Tarantini, and Dr. Andriy Yabluchanskiy serve as Associate Editors for GeroScience. The authors declare no competing financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rafal Gulej, Ádám Nyúl-Tóth, Chetan Ahire, and Jordan DelFaveroe have qual contributions.
About this article
Cite this article
Gulej, R., Nyúl-Tóth, Á., Ahire, C. et al. Elimination of senescent cells by treatment with Navitoclax/ABT263 reverses whole brain irradiation-induced blood-brain barrier disruption in the mouse brain. GeroScience 45, 2983–3002 (2023). https://doi.org/10.1007/s11357-023-00870-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00870-x