Abstract
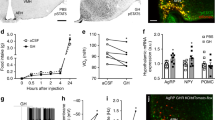
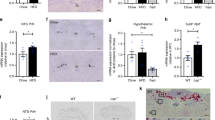
Evidence for hypothalamic regulation of energy homeostasis and thermoregulation in brown adipose tissue (BAT) during aging has been well recognized, yet the central molecular mediators involved in this process are poorly understood. The arcuate hypothalamus, orexigenic agouti–related peptide (AgRP) neurons control nutrient intake, energy homeostasis, and BAT thermogenesis. To determine the roles of growth hormone receptor (GHR) signaling in the AgRP neurons, we used mice with the AgRP-specific GHR deletion (AgRPΔGHR). We found that female AgRPΔGHR mice were resistant to temperature adaptation, and their body core temperature remained significantly lower when held at 10 °C, 22 °C, or 30 °C, compared to control mice. Low body core temperature in female AgRPΔGHR mice has been associated with significant reductions in Ucp1 and Pgc1α expression in the BAT. Further, neuronal activity in AgRP in response to cold exposure was blunted in AgRPΔGHR female mice, while the number of Fos+ AgRP neurons was increased in female controls exposed to cold. Global transcriptome from BAT identified increased the expression of genes related to immune responses and chemokine activity and decreased the expression of genes involved in triglyceride synthesis and metabolic pathways in AgRPΔGHR female mice. Importantly, these were the same genes that are downregulated by thermoneutrality in control mice but not in the AgRPΔGHR animals. Collectively, these data demonstrate a novel sex-specific role for GHR signaling in AgRP neurons in thermal regulation, which might be particularly relevant during aging.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AgRP:
-
Agouti-related peptide
- ARC:
-
Arcuate nucleus
- BAT:
-
Brown adipose tissue
- CNS:
-
Central nervous system
- DMH:
-
Dorsomedial hypothalamic nucleus
- GH:
-
Growth hormone
- GHR:
-
Growth hormone receptor
- GHRH:
-
Growth hormone–releasing hormone
- LHA:
-
Lateral hypothalamus
- POMC:
-
Proopiomelanocortin
- PVH:
-
Paraventricular hypothalamic nucleus
References
Mattson MP. Perspective: does brown fat protect against diseases of aging? Ageing Res Rev. 2010;9(1):69–76.
Roh E, Kim MS. Brain regulation of energy metabolism. Endocrinol Metab (Seoul). 2016;31(4):519–24.
Robertson SA, Leinninger GM, Myers MG Jr. Molecular and neural mediators of leptin action. Physiol Behav. 2008;94(5):637–42.
Belgardt BF, Bruning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci. 2010;1212:97–113.
Van Someren EJ. Thermoregulation and aging. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R99-102.
Osilla EV, JL Marsidi, S Sharma, Physiology, temperature regulation. StatPearls. 2022: Treasure Island (FL).
Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond). 2010;34(Suppl 1):S47-55.
Chong AC, Greendyk RA, Zeltser LM. Distinct networks of leptin- and insulin-sensing neurons regulate thermogenic responses to nutritional and cold challenges. Diabetes. 2015;64(1):137–46.
Han Y, et al. Deciphering an AgRP-serotoninergic neural circuit in distinct control of energy metabolism from feeding. Nat Commun. 2021;12(1):3525.
Yu S, et al. The hypothalamic preoptic area and body weight control. Neuroendocrinol. 2018;106(2):187–94.
Kuperman Y, et al. CRFR1 in AgRP neurons modulates sympathetic nervous system activity to adapt to cold stress and fasting. Cell Metab. 2016;23(6):1185–99.
Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225.
Waters MJ, et al. New insights into growth hormone action. J Mol Endocrinol. 2006;36(1):1–7.
Darcy J, et al. Brown adipose tissue function is enhanced in long-lived, male Ames dwarf mice. Endocrinol. 2016;157(12):4744–53.
Darcy J, et al. Increased environmental temperature normalizes energy metabolism outputs between normal and Ames dwarf mice. Aging (Albany NY). 2018;10(10):2709–22.
Westbrook R, et al. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64(4):443–51.
Furigo IC, et al. Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat Commun. 2019;10(1):662.
de Lima JBM, et al. Hypothalamic GHR-SIRT1 axis in fasting. Cells. 2021; 10(4).
List EO, et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinol. 2014;155(5):1793–805.
Sadagurski M, Cady G, Miller RA. Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell. 2017;16(4):652–60.
Sadagurski M, et al. Human IL6 enhances leptin action in mice. Diabetologia. 2010;53(3):525–35.
Schneider A, et al. Growth hormone signaling shapes the impact of environmental temperature on transcriptomic profile of different adipose tissue depots in male mice. J Gerontol: Series A. 2021;77(5):941–6.
Sanchez-Alavez M, Alboni S, Conti B. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age (Dordr). 2011;33(1):89–99.
Klein Hazebroek M, Keipert S. Adapting to the cold: a role for endogenous fibroblast growth factor 21 in thermoregulation? Front Endocrinol (Lausanne). 2020;11:389.
Deem JD, et al. Cold-induced hyperphagia requires AgRP neuron activation in mice. Elife. 2020;9.
Zimmer MR, et al. Functional ontogeny of hypothalamic Agrp neurons in neonatal mouse behaviors. Cell. 2019;178(1):44–59 (e7).
Timper K, Bruning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. 2017;10(6):679–89.
de Lima JBM, et al. ARCGHR neurons regulate muscle glucose uptake. Cells. 2021;10(5):1093.
Furigo IC, et al. Growth hormone enhances the recovery of hypoglycemia via ventromedial hypothalamic neurons. FASEB J. 2019; fj201901315R.
Wasinski F, et al. Growth hormone receptor deletion reduces the density of axonal projections from hypothalamic arcuate nucleus neurons. Neurosci. 2020;434:136–47.
Dietrich MO, et al. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell. 2015;160(6):1222–32.
Cavalcanti-de-Albuquerque JP, et al. Regulation of substrate utilization and adiposity by Agrp neurons. Nat Commun. 2019;10(1):311.
Graja A, Schulz TJ. Mechanisms of aging-related impairment of brown adipocyte development and function. Gerontol. 2015;61(3):211–7.
Burke LK, et al. mTORC1 in AGRP neurons integrates exteroceptive and interoceptive food-related cues in the modulation of adaptive energy expenditure in mice. Elife. 2017;6.
Dominick G, et al. Regulation of mTOR activity in Snell dwarf and GH receptor gene-disrupted mice. Endocrinol. 2015;156(2):565–75.
Campbell JN, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484–96.
Shi H, et al. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. Am J Physiol Endocrinol Metab. 2008;294(3):E630–9.
van de Wall E, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinol. 2008;149(4):1773–85.
Leung KC, et al. Estrogen regulation of growth hormone action. Endocr Rev. 2004;25(5):693–721.
Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci U S A. 2009;106(37):15932–7.
Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinol. 2002;75(5):296–305.
Morselli LL, et al. Control of energy expenditure by AgRP neurons of the arcuate nucleus: neurocircuitry, signaling pathways, and angiotensin. Curr Hypertens Rep. 2018;20(3):25.
Yan C, Chen J, Chen N. Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability. Sci Rep. 2016;6:22640.
Wang X, et al. Regulation of ANKRD9 expression by lipid metabolic perturbations. BMB Rep. 2009;42(9):568–73.
Yu XH, et al. LncRNA kcnq1ot1 promotes lipid accumulation and accelerates atherosclerosis via functioning as a ceRNA through the miR-452-3p/HDAC3/ABCA1 axis. Cell Death Dis. 2020;11(12):1043.
Ghimire S, Kim J. PEG3 controls lipogenesis through ACLY. PLoS One. 2021;16(5):e0252354.
Alshahrani S, et al. Increased Slc12a1 expression in beta-cells and improved glucose disposal in Slc12a2 heterozygous mice. J Endocrinol. 2015;227(3):153–65.
Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008;19(3):248–56.
Lee SH, et al. Lack of stearoyl-CoA desaturase 1 upregulates basal thermogenesis but causes hypothermia in a cold environment. J Lipid Res. 2004;45(9):1674–82.
Tran LT, et al. Hypothalamic control of energy expenditure and thermogenesis. Exp Mol Med. 2022;54(4):358–69.
Yasuda T, et al. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats. Neurosci Lett. 2003;349(2):75–8.
Nelson CN, et al. Growth hormone activated STAT5 is required for induction of beige fat in vivo. Growth Horm IGF Res. 2018;42–43:40–51.
Li Y, Knapp JR, Kopchick JJ. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Exp Biol Med (Maywood). 2003;228(2):207–15.
Desai BN, Harris RB. Integrated effects of leptin in the forebrain and hindbrain of male rats. Endocrinol. 2013;154(8):2663–75.
Hunter WS, et al. Low body temperature in long-lived Ames dwarf mice at rest and during stress. Physiol Behav. 1999;67(3):433–7.
Hauck SJ, et al. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med (Maywood). 2001;226(6):552–8.
Conti B, et al. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314(5800):825–8.
Waalen J, Buxbaum JN. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. J Gerontol A Biol Sci Med Sci. 2011;66(5):487–92.
Yanovich R, Ketko I, Charkoudian N. Sex differences in human thermoregulation: relevance for 2020 and beyond. Physiol. 2020;35(3):177–84.
Funding
This study was supported by the American Diabetes Association grant #1-lB-IDF-063, a Feasibility Grant from the Michigan Diabetes Research Center (P30DK020572) NIDDK, and MICP Core and metabolic core (P30DK020572) NIDDK and WSU funds for MS. LK was supported by NIH 5T32GM14519-01, and LS was supported by NIH 5T32HL120822-09.
Author information
Authors and Affiliations
Contributions
LS, JBML, and LKD carried out the research and reviewed the manuscript. MK and LK assisted in the data collection and experimental design. JJK contributed the GHR floxed mice. AS and AB analyzed the data and reviewed the manuscript. MS designed the study, analyzed the data, wrote the manuscript, and is responsible for the integrity of this work. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Stilgenbauer, L., de Lima, J.B.M., Debarba, L.K. et al. Growth hormone receptor (GHR) in AgRP neurons regulates thermogenesis in a sex-specific manner. GeroScience 45, 1745–1759 (2023). https://doi.org/10.1007/s11357-023-00726-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00726-4