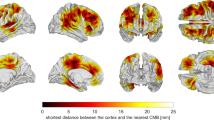
Abstract
Adults aged 60 and over are most vulnerable to mild traumatic brain injury (mTBI). Nevertheless, the extent to which chronological age (CA) at injury affects TBI-related brain aging is unknown. This study applies Gaussian process regression to T1-weighted magnetic resonance images (MRIs) acquired within \(\sim\)7 days and again \(\sim\)6 months after a single mTBI sustained by 133 participants aged 20–83 (CA \(\mu \pm \sigma\) = 42.6 ± 17 years; 51 females). Brain BAs are estimated, modeled, and compared as a function of sex and CA at injury using a statistical model selection procedure. On average, the brains of older adults age by 15.3 ± 6.9 years after mTBI, whereas those of younger adults age only by 1.8 ± 5.6 years, a significant difference (Welch’s t32 = − 9.17, p ≃ 9.47 × 10−11). For an adult aged \(\sim\)30 to \(\sim\)60, the expected amount of TBI-related brain aging is \(\sim\)3 years greater than in an individual younger by a decade. For an individual over \(\sim\)60, the respective amount is \(\sim\)7 years. Despite no significant sex differences in brain aging (Welch’s t108 = 0.78, p > 0.78), the statistical test is underpowered. BAs estimated at acute baseline versus chronic follow-up do not differ significantly (t264 = 0.41, p > 0.66, power = 80%), suggesting negligible TBI-related brain aging during the chronic stage of TBI despite accelerated aging during the acute stage. Our results indicate that a single mTBI sustained after age \(\sim\)60 involves approximately \(\sim\)10 years of premature and lasting brain aging, which is MRI detectable as early as \(\sim\)7 days post-injury.



Similar content being viewed by others
Abbreviations
- AG:
-
Age gap
- BA:
-
Biological age
- CA:
-
Chronological age
- HC:
-
Healthy control
- MRI:
-
Magnetic resonance imaging
- mTBI:
-
Mild traumatic brain injury
- OA:
-
Older adult
- YA:
-
Younger adult
References
Irimia A, et al. Statistical estimation of physiological brain age as a descriptor of senescence rate during adulthood. Brain Imaging Behav. 2015;9(4):678–89.
Cole JH, et al. Brain age and other bodily “ages”: implications for neuropsychiatry. Mol Psychiatry. 2019;24(2):266–81.
Franke K, Gaser C. Ten years of BrainAGE as a neuroimaging biomarker of brain aging: what insights have we gained? Front Neurol. 2019;10:789.
Faden AI, Loane DJ. Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics. 2015;12(1):143–50.
Irimia A, et al. Structural and connectomic neuroimaging for the personalized study of longitudinal alterations in cortical shape, thickness and connectivity after traumatic brain injury. J Neurosurg Sci. 2014;58(3):129–44.
de Freitas Cardoso MG, et al. Cognitive impairment following acute mild traumatic brain injury. Front Neurol. 2019;10:198.
Cole JH, et al. Brain age predicts mortality. Mol Psychiatry. 2018;23(5):1385–92.
Gaser C, et al. BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease. PLoS ONE. 2013;8(6): e67346.
Crane PK, et al. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 2016;73(9):1062–9.
Cole JH, et al. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage. 2017;163:115–24.
Cole JH, et al. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol. 2015;77(4):571–81.
Kliegel M, Martin M, Jager T. Development and validation of the Cognitive Telephone Screening Instrument (COGTEL) for the assessment of cognitive function across adulthood. J Psychol. 2007;141(2):147–70.
Tun PA, Lachman ME. Telephone assessment of cognitive function in adulthood: the Brief Test of Adult Cognition by Telephone. Age Ageing. 2006;35(6):629–32.
Team RC. R: A language and environment for statistical computing. 2013.
Karatzoglou A, et al. kernlab-an S4 package for kernel methods in R. J Stat Softw. 2004;11(1):1–20.
Williams CK. Prediction with Gaussian processes: from linear regression to linear prediction and beyond. In: Learning in graphical models. Springer; 1998. p. 599–621.
Beheshti I, et al. Bias-adjustment in neuroimaging-based brain age frameworks: a robust scheme. Neuroimage Clin. 2019;24: 102063.
de Lange AG, Cole JH. Commentary: Correction procedures in brain-age prediction. Neuroimage Clin. 2020;26: 102229.
Cook RD, Weisberg S. Characterizations of an empirical influence function for detecting influential cases in regression. Technometrics. 1980;22(4):495–508.
Rencher AC. Methods of multivariate analysis. 2nd ed. Wiley series in probability and mathematical statistics. New York: J. Wiley; 2002. xxii, 708 p.
Stram DO, Lee JW. Variance components testing in the longitudinal mixed effects model. Biometrics. 1994;50(4):1171–7.
Hox JJ, Moerbeek M, Van de Schoot R. Multilevel analysis: techniques and applications. Routledge; 2017.
Cnossen MC, et al. Adherence to guidelines in adult patients with traumatic brain injury: a living systematic review. J Neurotrauma. 2021;38(8):1072–85.
Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015;14(5):506–17.
Vanitallie TB. Parkinson disease: primacy of age as a risk factor for mitochondrial dysfunction. Metabolism. 2008;57(Suppl 2):S50–5.
Butterfield DA, Howard BJ, LaFontaine MA. Brain oxidative stress in animal models of accelerated aging and the age-related neurodegenerative disorders, Alzheimer’s disease and Huntington’s disease. Curr Med Chem. 2001;8(7):815–28.
Ho YS, et al. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS ONE. 2012;7(5): e36752.
Akyol MA, et al. Determining middle-aged and older adults’ health beliefs to change lifestyle and health behavior for dementia risk reduction. Am J Alzheimers Dis Other Demen. 2020;35:1533317519898996.
Lim YY, et al. Three-month stability of the CogState brief battery in healthy older adults, mild cognitive impairment, and Alzheimer’s disease: results from the Australian Imaging, Biomarkers, and Lifestyle-rate of change substudy (AIBL-ROCS). Arch Clin Neuropsychol. 2013;28(4):320–30.
Stephen R, et al. Change in CAIDE dementia risk score and neuroimaging biomarkers during a 2-year multidomain lifestyle randomized controlled trial: results of a post-hoc subgroup analysis. J Gerontol A Biol Sci Med Sci. 2021;76(8):1407–14.
Ishikawa Y, et al. Search for novel gene markers of traumatic brain injury by time differential microarray analysis. Acta Neurochir Suppl. 2006;96:163–7.
Liu R, et al. BpV(pic) confers neuroprotection by inhibiting M1 microglial polarization and MCP-1 expression in rat traumatic brain injury. Mol Immunol. 2019;112:30–9.
Pan MX, et al. Sex-dependent effects of GPER activation on neuroinflammation in a rat model of traumatic brain injury. Brain Behav Immun. 2020;88:421–31.
Wood RL. Accelerated cognitive aging following severe traumatic brain injury: a review. Brain Inj. 2017;31(10):1270–8.
Hicks AJ, et al. Does cognitive decline occur decades after moderate to severe traumatic brain injury? A prospective controlled study. Neuropsychol Rehabil. 2021;1–20.
Jackson CE, et al. Associations among increases in posttraumatic stress symptoms, neurocognitive performance, and long-term functional outcomes in U.S. Iraq War veterans. J Trauma Stress. 2021;34(3):628–40.
Mohamed AZ, et al. Traumatic brain injury fast-forwards Alzheimer's pathology: evidence from amyloid positron emission tomorgraphy imaging. J Neurol. 2021.
Toth L, et al. Traumatic brain injury-induced cerebral microbleeds in the elderly. Geroscience. 2021;43(1):125–36.
Hoare J, et al. Accelerated epigenetic aging in adolescents living with HIV is associated with altered development of brain structures. J Neurovirol. 2021.
McCartney DL, et al. Genome-wide association studies identify 137 genetic loci for DNA methylation biomarkers of aging. Genome Biol. 2021;22(1):194.
McCrory C, et al. GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. J Gerontol A Biol Sci Med Sci. 2021;76(5):741–9.
Voisin S, et al. An epigenetic clock for human skeletal muscle. J Cachexia Sarcopenia Muscle. 2020;11(4):887–98.
Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115.
Angelova DM, Brown DR. Microglia and the aging brain: are senescent microglia the key to neurodegeneration? J Neurochem. 2019;151(6):676–88.
Janowitz D, et al. Inflammatory markers and imaging patterns of advanced brain aging in the general population. Brain Imaging Behav. 2020;14(4):1108–17.
Vanni S, et al. Brain aging: a Ianus-faced player between health and neurodegeneration. J Neurosci Res. 2020;98(2):299–311.
Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci. 2015;66(Pt B):75–80.
Cho YE, et al. Older age results in differential gene expression after mild traumatic brain injury and is linked to imaging differences at acute follow-up. Front Aging Neurosci. 2016;8:168.
Gan S, et al. Accelerated brain aging in mild traumatic brain injury: longitudinal pattern recognition with white matter integrity. J Neurotrauma. 2021;38(18):2549–59.
Hawley C, et al. Traumatic brain injuries in older adults—6 years of data for one UK trauma centre: retrospective analysis of prospectively collected data. Emerg Med J. 2017;34(8):509–16.
Mosenthal AC, et al. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J Trauma. 2004;56(5):1042–8.
Centers for Disease, C. and Prevention. Trends in aging—United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52(6):101–4, 106.
Thompson HJ, Dikmen S, Temkin N. Prevalence of comorbidity and its association with traumatic brain injury and outcomes in older adults. Res Gerontol Nurs. 2012;5(1):17–24.
Lehallier B, et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019;25(12):1843–50.
Acknowledgements
The authors are thankful to Michelle Y. Ha, Dylan Overby, and Chur Tam for their comments, suggestions, assistance with literature search, and figure preparation. This study was supported by the National Institutes of Health grant R01 NS 100973 to A.I., by the US Department of Defense contract W81XWH-18-1-0413 to A.I., by a Hanson-Thorell Family Research Scholarship, and by the James J. and Sue Femino Foundation. The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Amgalan, A., Maher, A.S., Ghosh, S. et al. Brain age estimation reveals older adults’ accelerated senescence after traumatic brain injury. GeroScience 44, 2509–2525 (2022). https://doi.org/10.1007/s11357-022-00597-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00597-1