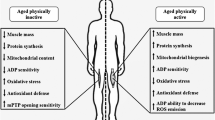
Abstract
Sarcopenia is a universal characteristic of the aging process and is often accompanied by increases in whole-body adiposity. These changes in body composition have important clinical implications, given that loss of muscle and gain of fat mass are both significantly and independently associated with declining physical performance as well as an increased risk for disability, hospitalizations, and mortality in older individuals. This increased fat mass is not exclusively stored in adipose depots but may become deposited in non-adipose tissues, such as skeletal muscle, when the oxidative capacity of the adipose tissue itself is exceeded. The redistributed adipose tissue is thought to exert detrimental local effects on the muscle environment given the close proximity. Thus, sarcopenia observed with aging may be better defined in the context of loss of muscle quality rather than loss of muscle quantity per se. In this perspective, we briefly review the age-related physiological changes in cellularity, secretory profiles, and inflammatory status of adipose tissue which drive lipotoxicity (spillover) of skeletal muscle and then provide evidence of how this may affect specific fiber type contractility. We focus on biological contributors (cellular machinery) to contractility for which there is some evidence of vulnerability to lipid stress distinguishing between fiber types.

Similar content being viewed by others
References
Ahn B, Ranjit R, Premkumar P, Pharaoh G, Piekarz KM, Matsuzaki S et al (2019) Mitochondrial oxidative stress impairs contractile function but paradoxically increases muscle mass via fibre branching. J Cachexia Sarcopenia Muscle 10(2):411–428. https://doi.org/10.1002/jcsm.12375
Akhmedov D, Berdeaux R (2013) The effects of obesity on skeletal muscle regeneration. Front Physiol 4:371. https://doi.org/10.3389/fphys.2013.00371
Albers PH, Pedersen AJT, Birk JB, Kristensen DE, Vind BF, Baba O et al (2015) Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes 64(2):485–497. https://doi.org/10.2337/db14-0590
Anderson EJ, Neufer PD (2006) Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am J Physiol Cell Physiol 290(3):C844–C851. https://doi.org/10.1152/ajpcell.00402.2005
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B et al (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479(7372):232–236. https://doi.org/10.1038/nature10600
Bandet CL, Tan-Chen S, Bourron O, Le Stunff H, Hajduch E (2019) Sphingolipid metabolism: new insight into ceramide-induced lipotoxicity in muscle cells. Int J Mol Sci 20(3). https://doi.org/10.3390/ijms20030479
Bollinger LM (2017) Potential contributions of skeletal muscle contractile dysfunction to altered biomechanics in obesity. Gait Posture 56:100–107. https://doi.org/10.1016/j.gaitpost.2017.05.003
Bosma M (2016) Lipid droplet dynamics in skeletal muscle. Exp Cell Res 340(2):180–186. https://doi.org/10.1016/j.yexcr.2015.10.023
Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA (2007) Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317(5839):807–810. https://doi.org/10.1126/science.1144090
Brown LA, Lee DE, Patton JF, Perry RA, Brown JL, Baum JI et al (2015) Diet-induced obesity alters anabolic signalling in mice at the onset of skeletal muscle regeneration. Acta Physiol 215(1):46–57. https://doi.org/10.1111/apha.12537
Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS et al (2010) Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev 9(4):369–383. https://doi.org/10.1016/j.arr.2010.04.004
Campisi J, d Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8(9):729–740. https://doi.org/10.1038/nrm2233
Chavez JA, Siddique MM, Wang ST, Ching J, Shayman JA, Summers SA (2014) Ceramides and glucosylceramides are independent antagonists of insulin signaling. J Biol Chem 289(2):723–734. https://doi.org/10.1074/jbc.M113.522847
Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK (2006) Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology 147(11):5340–5351. https://doi.org/10.1210/en.2006-0536
Ciapaite J, van den Berg SA, Houten SM, Nicolay K, van Dijk KW, Jeneson JA (2015) Fiber-type-specific sensitivities and phenotypic adaptations to dietary fat overload differentially impact fast- versus slow-twitch muscle contractile function in C57BL/6J mice. J Nutr Biochem 26(2):155–164. https://doi.org/10.1016/j.jnutbio.2014.09.014
Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E et al (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46(11):2347–2355. https://doi.org/10.1194/jlr.M500294-JLR200
Correa-de-Araujo R, Harris-Love MO, Miljkovic I, Fragala MS, Anthony BW, Manini TM (2017) The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: a symposium report. Front Physiol 8:87. https://doi.org/10.3389/fphys.2017.00087
D’Souza DM, Trajcevski KE, Al-Sajee D, Wang DC, Thomas M, Anderson JE, Hawke TJ (2015) Diet-induced obesity impairs muscle satellite cell activation and muscle repair through alterations in hepatocyte growth factor signaling. Physiol Rep 3(8). https://doi.org/10.14814/phy2.12506
de Wilde J, Mohren R, van den Berg S, Boekschoten M, Dijk KW-V, de Groot P et al (2008) Short-term high fat-feeding results in morphological and metabolic adaptations in the skeletal muscle of C57BL/6J mice. Physiol Genomics 32(3):360–369. https://doi.org/10.1152/physiolgenomics.00219.2007
Eshima H, Tamura Y, Kakehi S, Kurebayashi N, Murayama T, Nakamura K et al (2017) Long-term, but not short-term high-fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle. Physiol Rep (7):5. https://doi.org/10.14814/phy2.13250
Fisher AL (2004) Of worms and women: sarcopenia and its role in disability and mortality. J Am Geriatr Soc 52(7):1185–1190. https://doi.org/10.1111/j.1532-5415.2004.52320.x
Frier BC, Wan Z, Williams DB, Stefanson AL, Wright DC (2012) Epinephrine and AICAR-induced PGC-1α mRNA expression is intact in skeletal muscle from rats fed a high-fat diet. Am J Physiol Cell Physiol 302(12):C1772–C1779. https://doi.org/10.1152/ajpcell.00410.2011
Funai K, Lodhi IJ, Spears LD, Yin L, Song H, Klein S, Semenkovich CF (2016) Skeletal muscle phospholipid metabolism regulates insulin sensitivity and contractile function. Diabetes 65(2):358–370. https://doi.org/10.2337/db15-0659
Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB et al (2001) Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol 90(6):2157–2165
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV et al (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61(10):1059–1064. https://doi.org/10.1093/gerona/61.10.1059
Guo W, Pirtskhalava T, Tchkonia T, Xie W, Thomou T, Han J et al (2007) Aging results in paradoxical susceptibility of fat cell progenitors to lipotoxicity. Am J Physiol Endocrinol Metab 292(4):E1041–E1051. https://doi.org/10.1152/ajpendo.00557.2006
Harkins JM, Moustaid-Moussa N, Chung Y-J, Penner KM, Pestka JJ, North CM, Claycombe KJ (2004) Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr 134(10):2673–2677. https://doi.org/10.1093/jn/134.10.2673
Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259(5091):87–91. https://doi.org/10.1126/science.7678183
Janovská A, Hatzinikolas G, Mano M, Wittert GA (2010) The effect of dietary fat content on phospholipid fatty acid profile is muscle fiber type dependent. Am J Physiol Endocrinol Metab 298(4):E779–E786. https://doi.org/10.1152/ajpendo.00356.2009
Janssen I, Ross R (2005) Linking age-related changes in skeletal muscle mass and composition with metabolism and disease. J Nutr Health Aging 9(6):408–419
Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U (2007) Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev 128(1):36–44. https://doi.org/10.1016/j.mad.2006.11.008
Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB, Nicklas BJ (2018) Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci Med Sci 73(7):939–945. https://doi.org/10.1093/gerona/glx134
Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK et al (2019) Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40:554–563. https://doi.org/10.1016/j.ebiom.2018.12.052
Kaneko S, Iida R-H, Suga T, Fukui T, Morito M, Yamane A (2011) Changes in triacylglycerol-accumulated fiber type, fiber type composition, and biogenesis in the mitochondria of the soleus muscle in obese rats. Anat Rec 294(11):1904–1912. https://doi.org/10.1002/ar.21472
Kirkland JL (1992) The biochemistry of mammalian senescence. Clin Biochem 25(2):61–75
Kitessa SM, Abeywardena MY (2016) Lipid-induced insulin resistance in skeletal muscle: the chase for the culprit goes from total intramuscular fat to lipid intermediates, and finally to species of lipid intermediates. Nutrients 8(8). https://doi.org/10.3390/nu8080466
Lafontan M, Langin D (2009) Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48(5):275–297. https://doi.org/10.1016/j.plipres.2009.05.001
LeBrasseur NK, Tchkonia T, Kirkland JL (2015) Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr Inst Workshop Ser 83:11–18. https://doi.org/10.1159/000382054
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Mackrell JG, Arias EB, Cartee GD (2012) Fiber type-specific differences in glucose uptake by single fibers from skeletal muscles of 9- and 25-month-old rats. J Gerontol A Biol Sci Med Sci 67(12):1286–1294. https://doi.org/10.1093/gerona/gls194
McKiernan SH, Colman R, Lopez M, Beasley TM, Weindruch R, Aiken JM (2009) Longitudinal analysis of early stage sarcopenia in aging rhesus monkeys. Exp Gerontol 44(3):170–176. https://doi.org/10.1016/j.exger.2008.09.014
Meng J, Bencze M, Asfahani R, Muntoni F, Morgan JE (2015) The effect of the muscle environment on the regenerative capacity of human skeletal muscle stem cells. Skelet Muscle 5:11. https://doi.org/10.1186/s13395-015-0036-8
Miljkovic I, Zmuda JM (2010) Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care 13(3):260–264. https://doi.org/10.1097/MCO.0b013e328337d826
Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS (2001) Sarcopenia. J Lab Clin Med 137(4):231–243. https://doi.org/10.1067/mlc.2001.113504
Newman AB (2015) Is the onset of obesity the same as aging? Proc Natl Acad Sci U S A 112(52):E7163. https://doi.org/10.1073/pnas.1515367112
Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M et al (2003) Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition study. J Am Geriatr Soc 51(3):323–330
Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL et al (2009) Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging 1(9):771–783. https://doi.org/10.18632/aging.100075
Palmer AK, Kirkland JL (2016) Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp Gerontol 86:97–105. https://doi.org/10.1016/j.exger.2016.02.013
Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM et al (2019) Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18(3):e12950. https://doi.org/10.1111/acel.12950
Pinho RA, Sepa-Kishi DM, Bikopoulos G, Wu MV, Uthayakumar A, Mohasses A et al (2017) High-fat diet induces skeletal muscle oxidative stress in a fiber type-dependent manner in rats. Free Radic Biol Med 110:381–389. https://doi.org/10.1016/j.freeradbiomed.2017.07.005
Plomgaard P, Penkowa M, Pedersen BK (2005) Fiber type specific expression of TNF-alpha, IL-6 and IL-18 in human skeletal muscles. Exerc Immunol Rev 11:53–63
Poudyal H, Panchal SK, Ward LC, Brown L (2013) Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J Nutr Biochem 24(6):1041–1052. https://doi.org/10.1016/j.jnutbio.2012.07.014
Prakash YS, Sieck GC (1998) Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve 21(7):887–895. https://doi.org/10.1002/(SICI)1097-4598(199807)21:7<887::AID-MUS6>3.0.CO;2-2
Putti R, Migliaccio V, Sica R, Lionetti L (2015) Skeletal muscle mitochondrial bioenergetics and morphology in high fat diet induced obesity and insulin resistance: focus on dietary fat source. Front Physiol 6:426. https://doi.org/10.3389/fphys.2015.00426
Rai M, Demontis F (2016) Systemic nutrient and stress signaling via myokines and myometabolites. Annu Rev Physiol 78:85–107. https://doi.org/10.1146/annurev-physiol-021115-105305
Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM et al (2016) Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15(5):973–977. https://doi.org/10.1111/acel.12458
Roubenoff R, Castaneda C (2001) Sarcopenia-understanding the dynamics of aging muscle. J Am Med Assoc 286(10):1230–1231
Sakellariou GK, Lightfoot AP, Earl KE, Stofanko M, McDonagh B (2017) Redox homeostasis and age-related deficits in neuromuscular integrity and function. J Cachexia Sarcopenia Muscle 8(6):881–906. https://doi.org/10.1002/jcsm.12223
Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148(5):852–871. https://doi.org/10.1016/j.cell.2012.02.017
Sato C, Iso Y, Mizukami T, Otabe K, Sasai M, Kurata M et al (2016) Fibroblast growth factor-23 induces cellular senescence in human mesenchymal stem cells from skeletal muscle. Biochem Biophys Res Commun 470(3):657–662. https://doi.org/10.1016/j.bbrc.2016.01.086
Schafer MJ, White TA, Evans G, Tonne JM, Verzosa GC, Stout MB et al (2016) Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes 65(6):1606–1615. https://doi.org/10.2337/db15-0291
Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ et al (2017) Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8:14532. https://doi.org/10.1038/ncomms14532
Schilder RJ, Kimball SR, Marden JH, Jefferson LS (2011) Body weight-dependent troponin T alternative splicing is evolutionarily conserved from insects to mammals and is partially impaired in skeletal muscle of obese rats. J Exp Biol 214(Pt 9):1523–1532. https://doi.org/10.1242/jeb.051763
Schütze S, Wiegmann K, Machleidt T, Krönke M (1995) TNF-induced activation of NF-kappa B. Immunobiology 193(2–4):193–203
Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL (2011) Aging and regional differences in fat cell progenitors - a mini-review. Gerontology 57(1):66–75. https://doi.org/10.1159/000279755
Shaw CS, Jones DA, Wagenmakers AJM (2008) Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem Cell Biol 129(1):65–72. https://doi.org/10.1007/s00418-007-0349-8
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116(7):1793–1801. https://doi.org/10.1172/JCI29069
Shortreed KE, Krause MP, Huang JH, Dhanani D, Moradi J, Ceddia RB, Hawke TJ (2009) Muscle-specific adaptations, impaired oxidative capacity and maintenance of contractile function characterize diet-induced obese mouse skeletal muscle. PLoS One 4(10):e7293. https://doi.org/10.1371/journal.pone.0007293
Silvestri E, Cioffi F, De Matteis R, Senese R, de Lange P, Coppola M et al (2018) 3,5-Diiodo-L-thyronine affects structural and metabolic features of skeletal muscle mitochondria in high-fat-diet fed rats producing a co-adaptation to the glycolytic fiber phenotype. Front Physiol 9:194. https://doi.org/10.3389/fphys.2018.00194
Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJC, Parise G (2015) Satellite cells in human skeletal muscle plasticity. Front Physiol 6:283. https://doi.org/10.3389/fphys.2015.00283
Stearns-Reider KM, D’Amore A, Beezhold K, Rothrauff B, Cavalli L, Wagner WR et al (2017) Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 16(3):518–528. https://doi.org/10.1111/acel.12578
Stout MB, Justice JN, Nicklas BJ, Kirkland JL (2017) Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology 32(1):9–19. https://doi.org/10.1152/physiol.00012.2016
Stuart CA, McCurry MP, Marino A, South MA, Howell MEA, Layne AS et al (2013) Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J Clin Endocrinol Metab 98(5):2027–2036. https://doi.org/10.1210/jc.2012-3876
Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S et al (2007) Role of the toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 27(1):84–91. https://doi.org/10.1161/01.ATV.0000251608.09329.9a
Tchkonia T, Corkey BE, Kirkland JL (2006) Current views of the fat cell as an endocrine cell: lipotoxicity. In: Bray GA, Ryan DH (eds) Overweight and the metabolic syndrome. Springer US, Boston, pp 105–123. https://doi.org/10.1007/978-0-387-32164-6_6
Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H et al (2010) Fat tissue, aging, and cellular senescence. Aging Cell 9(5):667–684. https://doi.org/10.1111/j.1474-9726.2010.00608.x
Trim W, Turner JE, Thompson D (2018) Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Front Immunol 9:169. https://doi.org/10.3389/fimmu.2018.00169
Turpin SM, Lancaster GI, Darby I, Febbraio MA, Watt MJ (2006) Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab 291(6):E1341–E1350. https://doi.org/10.1152/ajpendo.00095.2006
Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HHCM, van Loon LJC (2007) Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292(1):E151–E157. https://doi.org/10.1152/ajpendo.00278.2006
Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJC (2014) Satellite cells in human skeletal muscle; from birth to old age. Age 36(2):545–547. https://doi.org/10.1007/s11357-013-9583-2
Visser M, Langlois J, Guralnik JM, Cauley JA, Kronmal RA, Robbins J et al (1998) High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr 68(3):584–590. https://doi.org/10.1093/ajcn/68.3.584
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–1808. https://doi.org/10.1172/JCI19246
Weiss R, Sachet M, Zinngrebe J, Aschacher T, Krainer M, Hegedus B et al (2013) IL-24 sensitizes tumor cells to TLR3-mediated apoptosis. Cell Death Differ 20(6):823–833. https://doi.org/10.1038/cdd.2013.15
Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA et al (2015) Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 4:e12997. https://doi.org/10.7554/eLife.12997
Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM et al (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med 24(8):1246–1256. https://doi.org/10.1038/s41591-018-0092-9
Zhang L, Morris KJ, Ng Y-C (2006) Fiber type-specific immunostaining of the Na+,K+-ATPase subunit isoforms in skeletal muscle: age-associated differential changes. Biochim Biophys Acta 1762(9):783–793. https://doi.org/10.1016/j.bbadis.2006.08.006
Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL (2014) Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 17(4):324–328. https://doi.org/10.1097/MCO.0000000000000065
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N et al (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14(4):644–658. https://doi.org/10.1111/acel.12344
Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F et al (2010) Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci 65(3):295–299. https://doi.org/10.1093/gerona/glp155
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Carter, C.S., Justice, J.N. & Thompson, L. Lipotoxicity, aging, and muscle contractility: does fiber type matter?. GeroScience 41, 297–308 (2019). https://doi.org/10.1007/s11357-019-00077-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-019-00077-z