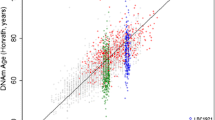
Abstract
DNA methylation (DNAm) has been found to show robust and widespread age-related changes across the genome. DNAm profiles from whole blood can be used to predict human aging rates with great accuracy. We sought to test whether DNAm-based predictions of age are related to phenotypes associated with type 2 diabetes (T2D), with the goal of identifying risk factors potentially mediated by DNAm. Our participants were 43 women enrolled in the Women’s Health Initiative. We obtained methylation data via the Illumina 450K Methylation array on whole blood samples from participants at three timepoints, covering on average 16 years per participant. We employed the method and software of Horvath, which uses DNAm at 353 CpGs to form a DNAm-based estimate of chronological age. We then calculated the epigenetic age acceleration, or Δage, at each timepoint. We fit linear mixed models to characterize how Δage contributed to a longitudinal model of aging and diabetes-related phenotypes and risk factors. For most participants, Δage remained constant, indicating that age acceleration is generally stable over time. We found that Δage associated with body mass index (p = 0.0012), waist circumference (p = 0.033), and fasting glucose (p = 0.0073), with the relationship with BMI maintaining significance after correction for multiple testing. Replication in a larger cohort of 157 WHI participants spanning 3 years was unsuccessful, possibly due to the shorter time frame covered. Our results suggest that DNAm has the potential to act as a mediator between aging and diabetes-related phenotypes, or alternatively, may serve as a biomarker of these phenotypes.


Similar content being viewed by others
References
Adalsteinsson BT et al (2012) Heterogeneity in white blood cells has potential to confound DNA methylation measurements. PLoS One 7:e46705. https://doi.org/10.1371/journal.pone.0046705
Alisch RS, Barwick BG, Chopra P, Myrick LK, Satten GA, Conneely KN, Warren ST (2012) Age-associated DNA methylation in pediatric populations. Genome Res 22:623–632. https://doi.org/10.1101/gr.125187.111
Association AD (2016) Erratum. Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes-2016. Diabetes Care 2016;39(Suppl. 1):S13-S22 Diabetes Care 39:1653 doi:https://doi.org/10.2337/dc16-er09
Baker GT, Sprott RL (1988) Biomarkers of aging Exp Gerontol 23:223–239 doi:https://doi.org/10.1016/0531-5565(88)90025-3
Barfield RT, Kilaru V, Smith AK, Conneely KN (2012) CpGassoc: an R function for analysis of DNA methylation microarray data. Bioinformatics 28:1280–1281. https://doi.org/10.1093/bioinformatics/bts124
Bollati V et al (2009) Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev 130:234–239. https://doi.org/10.1016/j.mad.2008.12.003
Breitling PL, Saum K-U, Perna L, Schöttker B, Holleczek B (2016) Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin Epigenetics:8.1: 21. https://doi.org/10.1186/s13148-016-0186-5
Burch JB et al (2014) Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S1–S3. https://doi.org/10.1093/gerona/glu041
Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC (1994) Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17:961–969
Chen BH et al (2016) DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY) 8:1844–1865. https://doi.org/10.18632/aging.101020
Christensen BC et al (2009) Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet 5:e1000602. https://doi.org/10.1371/journal.pgen.1000602
Christiansen L, Lenart A, Tan Q, Vaupel JW, Aviv A, McGue M, Christensen K (2016) DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell 15:149–154. https://doi.org/10.1111/acel.12421
Colberg SR et al (2010) Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 42:2282–2303. https://doi.org/10.1249/MSS.0b013e3181eeb61c
Fagnoni FF et al (2000) Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood 95:2860–2868
International Diabetes Federation (2015) IDF Diabetes Atlas, 7th edn. Brussels, Belgium: International Diabetes Federation
Feinberg AP (2007) Phenotypic plasticity and the epigenetics of human disease. Nature 447:433–440. https://doi.org/10.1038/nature05919
Ford ES, Williamson DF, Liu S (1997) Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 146:214–222
Goldberg AD, Allis CD, Bernstein E (2007) Epigenetics: a landscape takes shape. Cell 128:635–638. https://doi.org/10.1016/j.cell.2007.02.006
The Women’s Health Initiative Study Group (1998) Design of the Women’s Health Initiative clinical trial and observational study. Controlled Clin Trials 19.1 (1998): 61–109
Hannum G et al (2013) Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell 49:359–367. https://doi.org/10.1016/j.molcel.2012.10.016
Hays J et al. (2003) The Women’s Health Initiative recruitment methods and results. Ann Epidemiol 13.9 (2003): S18–S77
He W, D. Goodkind, P. Kowal (2016) An aging world: 2015. International Population Reports P95/09–1
Heinemann L (2010) Insulin assay standardization: leading to measures of insulin sensitivity and secretion for practical clinical care response to Staten et al. Diabetes Care 33:e83. https://doi.org/10.2337/dc10-0034
Hidalgo B et al (2014) Epigenome-wide association study of fasting measures of glucose, insulin, and HOMA-IR in the genetics of lipid lowering drugs and diet network study. Diabetes 63:801–807. https://doi.org/10.2337/db13-1100
Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14:R115. https://doi.org/10.1186/gb-2013-14-10-r115
Horvath S et al (2015) Decreased epigenetic age of PBMCs from Italian semi-supercentenarians and their offspring. Aging (Albany NY) 7:1159–1170. 10.18632/aging.100861
Horvath S et al (2016) An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol 17:171. https://doi.org/10.1186/s13059-016-1030-0
Houseman EA et al (2012) DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 13:86. https://doi.org/10.1186/1471-2105-13-86
Hu FB (2011) Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34:1249–1257. https://doi.org/10.2337/dc11-0442
Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC (2001) Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 345:790–797. https://doi.org/10.1056/NEJMoa010492
Johnson TE (2006) Recent results: biomarkers of aging. Exp Gerontol 41:1243–1246. https://doi.org/10.1016/j.exger.2006.09.006
Kaeberlein M, Rabinovitch PS, Martin GM (2015) Healthy aging: the ultimate preventative medicine. Science 350:1191–1193. https://doi.org/10.1126/science.aad3267
Kananen L et al (2016) The trajectory of the blood DNA methylome ageing rate is largely set before adulthood: evidence from two longitudinal studies. Age (Dordr) 38:65. https://doi.org/10.1007/s11357-016-9927-9
Kennedy BK et al (2014) Geroscience: linking aging to chronic disease. Cell 159:709–713. https://doi.org/10.1016/j.cell.2014.10.039
Koh-Banerjee P, Wang Y, FB H, Spiegelman D, Willett WC, Rimm EB (2004) Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol 159:1150–1159. https://doi.org/10.1093/aje/kwh167
Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD (2012) The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28:882–883. https://doi.org/10.1093/bioinformatics/bts034
Levine M et al. (2015) DNA methylation age of blood predicts future onset of lung cancer in the Women’s Health Initiative. Aging (Albany NY) 7:690-700. https://doi.org/10.18632/aging.100809
Levine ME et al (2016) Menopause accelerates biological aging. Proc Natl Acad Sci U S A 113:9327–9332. https://doi.org/10.1073/pnas.1604558113
Marioni RE et al (2015a) DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol 16:25. https://doi.org/10.1186/s13059-015-0584-6
Marioni RE et al (2015b) The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol 44:1388–1396. https://doi.org/10.1093/ije/dyu277
McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G (2003) Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med 139:802–809. https://doi.org/10.7326/0003-4819-139-10-200311180-00007
Mendelson MM et al (2017) Association of body mass index with DNA methylation and gene expression in blood cells and relations to cardiometabolic disease: a Mendelian randomization approach. PLoS Med 14:e1002215. https://doi.org/10.1371/journal.pmed.1002215
Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS (2003) Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289:76–79. https://doi.org/10.1001/jama.289.1.76
Nathan DM et al (2007) Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30:753–759. https://doi.org/10.2337/dc07-9920
Nevalainen T et al (2017) Obesity accelerates epigenetic aging in middle-aged but not in elderly individuals. Clin Epigenetics 9:20. https://doi.org/10.1186/s13148-016-0301-7
Niccoli T, Partridge L (2012) Ageing as a risk factor for disease. Curr Biol 22:R741–R752. https://doi.org/10.1016/j.cub.2012.07.024
Nilsson E et al (2014) Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes 63:2962–2976. https://doi.org/10.2337/db13-1459
Pan XR et al (1997) Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 20:537–544
Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T (1999) Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol 9:178–187
Perna L, Zhang Y, Mons U, Holleczek B, Saum KU, Brenner H (2016) Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin Epigenetics 8:64. https://doi.org/10.1186/s13148-016-0228-z
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC (2016) nlme: linear and nonlinear mixed effects models. vol 3.1–128. R package version
Quach A et al (2017) Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany NY) 9:419-446. https://doi.org/10.18632/aging.101168
Reinius LE et al (2012) Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One 7:e41361. https://doi.org/10.1371/journal.pone.0041361
Ronn T, Ling C (2015) DNA methylation as a diagnostic and therapeutic target in the battle against type 2 diabetes. Epigenomics 7:451–460. https://doi.org/10.2217/epi.15.7
Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA, FB H (2006) Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care 29:1585–1590. https://doi.org/10.2337/dc06-0057
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14. https://doi.org/10.1016/j.diabres.2009.10.007
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F (2008) The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 6:299–304. https://doi.org/10.1089/met.2008.0034
Stamler J, Vaccaro O, Neaton JD, Wentworth D (1993) Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care 16:434–444
Teschendorff AE et al (2010) Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res 20:440–446. https://doi.org/10.1101/gr.103606.109
Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, Beck S (2013) A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 29:189–196. https://doi.org/10.1093/bioinformatics/bts680
Wahl S et al (2017) Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 541:81–86. https://doi.org/10.1038/nature20784
Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE (2000) A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes 49:2094–2101. https://doi.org/10.2337/diabetes.49.12.2094
Xu Z, Taylor JA (2014) Genome-wide age-related DNA methylation changes in blood and other tissues relate to histone modification, expression and cancer. Carcinogenesis 35:356–364. https://doi.org/10.1093/carcin/bgt391
Yokoyama H et al (2003) Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diabetes Care 26:2426–2432. https://doi.org/10.2337/diacare.26.8.2426
Zheng Y et al (2016) Blood epigenetic age may predict cancer incidence and mortality. EBioMedicine 5:68–73. https://doi.org/10.1016/j.ebiom.2016.02.008
Funding
We gratefully acknowledge funding from “Longitudinal study of DNA methylation as a mediator between age and cardiovascular risk” (National Heart, Lung, and Blood Institute (NHLBI) Contract #HHSN268201100046C, to K.C.) and “Epigenetic Mechanisms of PM-Mediated CVD Risk” (National Institute of Environmental Health Sciences (NIEHS) R01-ES020836, to E.W.), P30ES009089 to A.B., National Institute on Aging (NIA) U34-AG051418 to K.C. and A.B., and DGE-1444932 (National Science Foundation Graduate Research Fellowships Program (NSF GRFP), to C.G.). This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. (DGE-1444932). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. All contributors to WHI science are listed at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 33842 kb)
About this article
Cite this article
Grant, C.D., Jafari, N., Hou, L. et al. A longitudinal study of DNA methylation as a potential mediator of age-related diabetes risk. GeroScience 39, 475–489 (2017). https://doi.org/10.1007/s11357-017-0001-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-017-0001-z