Abstract
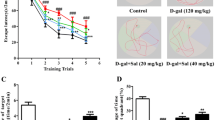
Cognitive deficiency and oxidative stress have been well documented in aging and in neurodegenerative disorders such as Alzheimer’s disease. In this study, we assessed the therapeutic effect of polyprenols on d-galactose-induced cognitive impairment in mice by testing on of behavioral and cognitive performance. In order to explore the possible role of polyprenols against d-galactose-induced oxidative damages, we assessed various biochemical indicators. Chronic administration of d-galactose (150 mg/kg·d, s.c.) for 7 weeks significantly impaired cognitive performance (both in step-through passive and active avoidance tests) and locomotor activity (in open-field test) and the ability of spatial learning and memory (in Morris water maze test) compared with the control group. The results revealed that polyprenols treatment for 2 weeks significantly ameliorated model mice’s cognitive performance and oxidative defense. All groups of polyprenols enhanced the learning and memory ability in step-through passive and active avoidance tests, locomotor activity in open-field test, and the ability of spatial learning and memory in Morris water maze test. Furthermore, high and middle level of polyprenols significantly increased total antioxidative capacity (T-AOC), glutathione peroxidase (GSH-Px), super oxide dismutase (SOD) activity, neprilysin (NEP), and β-site AβPP cleaving enzyme 1 (BACE1) expression, while nitric oxide (NO), nitric oxide synthase (NOS) activity, malondialdehyde (MDA) concentration, and the level of Aβ1-42 and presenilin 1 (PS1) were decreased. Polyprenols have a significant relieving effect on learning, memory, and spontaneous activities in a d-galactose-induced mouse model and ameliorates cognitive impairment and biochemical dysfunction in mice. In summary, we have demonstrated that polyprenols may ameliorate memory and cognitive impairment via enhancing oxidative defense and affecting generation and dissimilation of Aβ-related enzymes, suggesting that polyprenols represent a novel drug for treating Alzheimer’s disease.







Similar content being viewed by others
References
Briganti S, Wlaschek M, Hinrichs C (2008) Small molecular antioxidants effectively protect from PUVA-induced oxidative stress responses underlying fibroblast senescence and photoaging. Free Radic Biol Med 45:636–644
Choi D-Y, Lee Y-J, Hong JT et al (2012) Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res Bull 87:144–153
Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J et al (2006) Chronic systemic d-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res 83:1584–1590
DaRocha-Souto B, Coma M, Pérez-Nievas BG (2012) synthase kinase-3 beta mediates β-amyloid induced neuritic damage in AD. Neurobiol Dis 45:425–437
Dominique BR, Christelle CA, Antonio T (2004) Blood oxidative stress status in patients with macrophagic myofasciitis. Biomed Pharmacother 58(9):516–519
Dumont M, Beal MF (2011) Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic Biol Med 51:1014–1026
Gubandru M, Margina D, Tsitsimpikou C (2013) Alzheimer’s disease treated patients showed different patterns for oxidative stress and inflammation markers. Food Chem Toxicol. doi:10.1016/j.fct.2013.07.013
He M, Zhao L, Wei MJ, Yao WF, Zhao HS, Chen FJ (2009) Neuroprotective effects of (−)-epigallocatechin-3-gallate on aging mice induced by d-galactose. Biol Pharm Bull 32:55–60
Kwon SH, Lee HK (2011) Neuroprotective effects of Eucommia ulmoides Oliv. Bark on amyloid beta 25–35-induced learning and memory impairments in mice. Neurosci Lett 487:123–127
Lei M, Su Y, Hua X, Ding J, Han Q, Hu G et al (2008) Chronic systemic injection of D -galactose impairs the septohippocampal cholinergic system in rats. Neuroreport 19:1611–1615
Lei M, Zhu Z, Wen Z, Ke S (2013) Impairments of tight junctions are involved in d-galactose-induced brain aging. Neuroreport 24(12):671–676
Long J, Wang X, Gao H, Liu Z, Liu C, Miao M et al (2007) d-galactose toxicity in mice is associated with mitochondrial dysfunction: protecting effects of mitochondrial nutrient R-alpha-lipoic acid. Biogerontology 8:373–381
Lu J, Zheng YL, Luo L, Wu DM, Sun DX, Feng YJ (2006) Quercetin reverses d-galactose induced neurotoxicity in mouse brain. Behav Brain Res 171:251–260
Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Ye Q et al (2010) Ursolic acid attenuates d-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-kB pathway activation. Cereb Cortex 20:2540–2548
Malito E, Ralat LA, Manolopoulou M et al (2008) Molecular bases for the recognition of short peptide substrates and cysteine-directed modifications of human insulin-degrading enzyme. Biochemistry 47:12822–12834
Marchei P, Diverio S, Falocci NC (2009) Breed differences in behavioural development in kittens. Physiol Behav 96(4):522–531
Marseille DM, Silverman DHS (2006) Recognition and treatment of Alzheimer’s disease: a case-based review. Am J Alzheimers Dis Other Dement 21:119–125
Naghizadeh B, Mansouri MT, Ghorbanzadeh B (2013) Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine 20:537–542
Nunomura A, Perry G, Pappolla MA (1999) RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci 19(6):1959–1964
Nunomura A, Perry G, Aliev G (2001) Oxidative damage is the earliest event in Alzheimer disease. Neuropathol Exp Neurol 60(8):759–767
Ozdemir E, Cetinkaya S, Ersan S (2009) Serum selenium and plasma malondialdehyde levels and antioxidant enzyme activities in patients with obsessive-compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 33:62–65
Pamplona R, Dalfó E, Ayala V (2005) Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Effects of Alzheimer's disease and identification of lipoxidation targets. J Biol Chem 280(22):21522–21530
Pan Y, Chen Y, Li Q, Yu X, Wang J, Zheng J (2013) The synthesis and evaluation of novel hydroxyl substituted chalcone analogs with in vitro anti-free radicals pharmacological activity and in vivo anti-oxidation activity in a free radical-injury Alzheimer’s model. Molecules 18:1693–1703
Soto C, Branes M, Alvarez J (1994) Structural determinants of the Alzheimer’s amyloid beta-peptide. J Neurochem 63(4):1191–1198
Tian J, Shi J, Yin J et al (2009) GEPT extract reduces Abeta deposition by regulating the balance between production and degradation of Abeta in APPV717I transgenic mice. Curr Alzheimers Res 6:118–131
Valls V, Peiro C, Muniz P (2005) Age-related changes in antioxidant status and oxidative damage to lipids and DNA in mitochondria of rat liver. Process Biochem 40(2):903–908
Vanaja P, Ekambaram P (2004) Demonstr ating the dose- and time-related effects of 7-nitroindazole on picrotoxin-induc ed convulsions, memory formation, brain nitric oxide synthase activity, and nitric oxide concentration in rats. Pharmacol Biochem Behav 77:1–8
Wang CM, Liu MY, Wang F (2013) Anti-amnesic effect of pseudoginsenoside-F11 in two mouse models of Alzheimer’s disease. Pharmacol Biochem Behav 106:57–67
Weitzdoerfer R, Hoeger H, Engidawork E (2004) Neuronal nitric oxide synthase knoekout mice show impaired cognitive performance. Nitric Oxide 10:130–140
Wolfe MS (2012) Processive proteolysis by γ-secretase and the mechanism of Alzheimer’s disease. Biol Chem 393:899–905
Xian YF, Lin ZX, Zhao M, Mao QQ, Ip SP, Che CT (2011) Uncaria rhynchophylla ameliorates cognitive deficits induced by d-galactose in mice. Planta Med 77:1977–1983
Yang J, He L, Wang J (2004) Early administration of nicotinamide prevents learning and memory impairment in mice induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Pharmacol Biochem Behav 78:179–183
Yu J, Wang Y, Qian H, Zhao Y, Liu B, Fu C et al (2012) Polyprenols from Taxus chinensis var. mairei prevent the development of CCl4-induced liver fibrosis in rats. J Ethnopharmacol 142(1):151–160
Zheng GY, HE L, BO CY, Wang MM (2011) Protective effect of polyprenols from pine needles of Pinus massoniana on damaged PC12cells injury induced by β-amyloid protein, Chinese. Pharmacol Bull 27:581–582
Acknowledgments
This study was supported by the National 12th Five-Year Plan “Major Scientific and Technological Special Project for Significant New Drugs Creation” project of “Novel G protein-coupled receptor targeted drug screening system and key technology research” (no. 2012ZX09504001-001); Program for New Century Excellent Talents in University (no. NCET-10-0817); Major Scientific and Technological Special Project of Guangdong Province (no. 2012A080201005); and the Fundamental Research Funds for the Central Universities (no. JKZ2009005 and no. JKY2011052).
Conflicts of interest
These authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wang, C., He, L., Yan, M. et al. Effects of polyprenols from pine needles of Pinus massoniana on ameliorating cognitive impairment in a d-galactose-induced mouse model. AGE 36, 9676 (2014). https://doi.org/10.1007/s11357-014-9676-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-014-9676-6