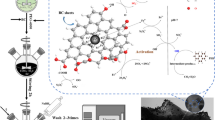
Abstract
In this study, boron-doped porous carbon materials (BCs) with high surface areas were synthesized employing coffee grounds as carbon source and sodium bicarbonate and boric acid as precursors; afterward, nanoscale zero-valent iron (nZVI) and BCs composites (denoted as nZVI@BCs) were further prepared through reduction of FeSO4 by NaBH4 along with stirring. The performance of the nZVI@BCs for activating persulfate (PS) was evaluated for the degradation of bisphenol A (BPA). In comparison with nZVI@Cs/PS, nZVI@BCs/PS could greatly promote the degradation and mineralization of BPA via both radical and non-radical pathways. On the one hand, electron spin resonance and radical quenching studies represented that •OH, SO4•−, and O2•− were mainly produced in the nZVI@BCs/PS system for BPA degradation. On the other hand, the open circuit voltages of nZVI@BCs and nZVI@Cs in different systems indicated that non-radical pathway still existed in our system. PS could grab the unstable unpaired electron on nZVI@BCs to form a carbon material surface-confined complex ([nZVI@BCs]*) with a high redox potential, then accelerate BPA removal efficiency via direct electron transfer. Furthermore, the performances and mechanisms for BPA degradation were examined by PS activation with nZVI@BC composites at various conditions including dosages of nZVI@BCs, BPA and PS, initially pH value, temperature, common anions, and humid acid. Therefore, this study provides a novel insight for development of high-performance carbon catalysts toward environmental remediation.









Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Barzegar G, Jorfi S, Zarezade V, Khatebasreh M, Mehdipour F, Ghanbari F (2018) 4-Chlorophenol degradation using ultrasound/peroxymonosulfate/nanoscale zero valent iron: reusability, identification of degradation intermediates and potential application for real wastewater. Chemosphere 201:370–379
Chen C, Ma T, Shang Y, Gao B, Jin B, Dan H, Li Q, Yue Q, Li Y, Wang Y, Xu X (2019) In-situ pyrolysis of Enteromorpha as carbocatalyst for catalytic removal of organic contaminants: considering the intrinsic N/Fe in Enteromorpha and non-radical reaction. Appl Catal B 250:382–395
Cheng X, Guo H, Zhang Y, Wu X, Liu Y (2017) Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res 113:80–88
Cheng X, Guo H, Zhang Y, Korshin GV, Yang B (2019) Insights into the mechanism of nonradical reactions of persulfate activated by carbon nanotubes: activation performance and structure-function relationship. Water Res 157:406–414
Cheng X, Huo X, Yang B, Li W, Wang Q, Zhang Y (2022) Deprivation of unpaired electrons on graphitic carbon nitride-based carbocatalysts by peroxydisulfate driving a nonradical oxidation process. J Clean Prod 334:130220
Dong H, He Q, Zeng G, Tang L, Zhang L, Xie Y, Zeng Y, Zhao F (2017) Degradation of trichloroethene by nanoscale zero-valent iron (nZVI) and nZVI activated persulfate in the absence and presence of EDTA. Chem Eng J 316:410–418
Duan X, Sun H, Kang J, Wang Y, Indrawirawan S, Wang S (2015) Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons. ACS Catal 5:4629–4636
Duan X, Ao Z, Li D, Sun H, Zhou L, Suvorova A, Saunders M, Wang G, Wang S (2016a) Surface-tailored nanodiamonds as excellent metal-free catalysts for organic oxidation. Carbon 103:404–411
Duan X, Su C, Zhou L, Sun H, Suvorova A, Odedairo T, Zhu Z, Shao Z, Wang S (2016b) Surface controlled generation of reactive radicals from persulfate by carbocatalysis on nanodiamonds. Appl Catal B-Environ 194:7–15
Duan X, Sun H, Wang S (2018) Metal-free carbocatalysis in advanced oxidation reactions. Acc Chem Res 51:678–687
Gao C, Yu W, Zhu Y, Wang M, Tang Z, Du L, Hu M, Fang L, Xiao X (2021) Preparation of porous silicate supported micro-nano zero-valent iron from copper slag and used as persulfate activator for removing organic contaminants. Sci Total Environ 754:142131
Ghauch A, Tuqan AM (2012) Oxidation of bisoprolol in heated persulfate/H2O systems: kinetics and products. Chem Eng J 183:162–171
Huo X, Liu Y, Liu S, Zhou P, Li H, Yang B, Yang D, Zhang Y (2019) Coupled removal of rhodamine B and Cu2+ by activating persulfate using micron zero-valent iron. J Water Supply Res Technol AQUA 68:535–546
Huo X, Zhou P, Zhang J, Liu Y, Cheng X, Liu Y, Li W, Zhang Y (2020) N, S-doped porous carbons for persulfate activation to remove tetracycline: nonradical mechanism. J Hazard Mater 391:122055
Ji H, Zhu Y, Duan J, Liu W, Zhao D (2019) Reductive immobilization and long-term remobilization of radioactive pertechnetate using bio-macromolecules stabilized zero valent iron nanoparticles. Chin Chem Lett 30:2163–2168
Lee H, Kim H-i, Weon S, Choi W, Hwang YS, Seo J, Lee C, Kim J-H (2016) Activation of persulfates by graphitized nanodiamonds for removal of organic compounds. Environ Sci Technol 50:10134–10142
Li R, Jin X, Megharaj M, Naidu R, Chen Z (2015) Heterogeneous Fenton oxidation of 2,4-dichlorophenol using iron-based nanoparticles and persulfate system. Chem Eng J 264:587–594
Li Z, Sun Y, Yang Y, Han Y, Wang T, Chen J, Tsang DCW (2020) Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J Hazard Mater 383:121240
Liang C, Su H-W (2009) Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind Eng Chem Res 48:5558–5562
Lin K-YA, Lin J-T, Lu X-Y, Hung C, Lin Y-F (2017) Electrospun magnetic cobalt-embedded carbon nanofiber as a heterogeneous catalyst for activation of oxone for degradation of Amaranth dye. J Colloid Interface Sci 505:728–735
Lin Y, Wu S, Yang C, Chen M, Li X (2019) Preparation of size-controlled silver phosphate catalysts and their enhanced photocatalysis performance via synergetic effect with MWCNTs and PANI. Appl Catal B 245:71–86
Liu Z, Zhang F-S, Wu J (2010) Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 89:510–514
Luo R, Li M, Wang C, Zhang M, Khan MAN, Sun X, Shen J, Han W, Wang L, Li J (2019) Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition. Water Res 148:416–424
Oh S-Y, Kim H-W, Park J-M, Park H-S, Yoon C (2009) Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J Hazard Mater 168:346–351
Peng W, Liu S, Sun H, Yao Y, Zhi L, Wang S (2013) Synthesis of porous reduced graphene oxide as metal-free carbon for adsorption and catalytic oxidation of organics in water. J Mater Chem A 1:5854–5859
Pulicharla R, Drouinaud R, Brar SK, Drogui P, Proulx F, Verma M, Surampalli RY (2018) Activation of persulfate by homogeneous and heterogeneous iron catalyst to degrade chlortetracycline in aqueous solution. Chemosphere 207:543–551
Ren W, Xiong L, Nie G, Zhang H, Duan X, Wang S (2020) Insights into the electron-transfer regime of peroxydisulfate activation on carbon nanotubes: the role of oxygen functional groups. Environ Sci Technol 54:1267–1275
Song Y, Fang G, Zhu C, Zhu F, Wu S, Chen N, Wu T, Wang Y, Gao J, Zhou D (2019) Zero-valent iron activated persulfate remediation of polycyclic aromatic hydrocarbon-contaminated soils: an <i>in situ</i> pilot-scale study. Chem Eng J 355:65–75
Tang L, Liu Y, Wang J, Zeng G, Deng Y, Dong H, Feng H, Wang J, Peng B (2018) Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl Catal B-Environ 231:1–10
Tian W, Zhang H, Duan X, Sun H, Tade MO, Ang HM, Wang S (2016) Nitrogen- and sulfur-codoped hierarchically porous carbon for adsorptive and oxidative removal of pharmaceutical contaminants. ACS Appl Mater Interfaces 8:7184–7193
Wang X, Qin Y, Zhu L, Tang H (2015) Nitrogen-doped reduced graphene oxide as a bifunctional material for removing bisphenols: synergistic effect between adsorption and catalysis. Environ Sci Technol 49:6855–6864
Wang Y, Chen S-y, Yang X, Huang X-f, Yang Y-h, He E-k, Wang S, Qiu R-l (2017) Degradation of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) by a nano zerovalent iron-activated persulfate process: the effect of metal ions. Chem Eng J 317:613–622
Wu S, He H, Li X, Yang C, Zeng G, Wu B, He S, Lu L (2018a) Insights into atrazine degradation by persulfate activation using composite of nanoscale zero-valent iron and graphene: Performances and mechanisms. Chem Eng J 341:126–136
Wu S, Li H, Li X, He H, Yang C (2018b) Performances and mechanisms of efficient degradation of atrazine using peroxymonosulfate and ferrate as oxidants. Chem Eng J 353:533–541
Wu S, Liu H, Lin Y, Yang C, Lou W, Sun J, Du C, Zhang D, Nie L, Yin K, Zhong Y (2020) Insights into mechanisms of UV/ferrate oxidation for degradation of phenolic pollutants: role of superoxide radicals. Chemosphere 244:UNSP125450
Xu B, Jiang W, Wang L, Thokchom B, Qiu P, Luo W (2020) Yolk-shell structured Fe@void@mesoporous silica with high magnetization for activating peroxymonosulfate. Chin Chem Lett 31:2003–2006
Yang B, Zhou P, Cheng X, Li H, Huo X, Zhang Y (2019) Simultaneous removal of methylene blue and total dissolved copper in zero-valent iron/H2O2 Fenton system: kinetics, mechanism and degradation pathway. J Colloid Interface Sci 555:383–393
Yi H, Huo X, Gu J, Wei L, Sun Z, Du F, Dai C, Wu X, Liu Z, Ren J (2022) Boron doping positively enhances the catalytic activity of carbon materials for the removal of bisphenol A. RSC Adv 12:21780–21792
Zhang Y, Zhang J, Xiao Y, Chang VWC, Lim T-T (2016) Kinetic and mechanistic investigation of azathioprine degradation in water by UV, UV/H 2 O 2 and UV/persulfate. Chem Eng J 302:526–534
Zhao L, Ji Y, Kong D, Lu J, Zhou Q, Yin X (2016) Simultaneous removal of bisphenol A and phosphate in zero-valent iron activated persulfate oxidation process. Chem Eng J 303:458–466
Zhou P, Li W, Zhang J, Zhang G, Cheng X, Liu Y, Huo X, Zhang Y (2019) Removal of rhodamine B during the corrosion of zero valent tungsten via a tungsten species-catalyzed Fenton-like system. J Taiwan Inst Chem Eng 100:202–209
Zhou P, Zhang J, Xiong Z, Liu Y, Huo X, Cheng X, Li W, Cheng F, Zhang Y (2020) C-60 Fullerol promoted Fe(III)/H2O2 Fenton oxidation: role of photosensitive Fe(III)-Fullerol complex. Appl Catal B-Environ 265:118264
Zhu S, Huang X, Ma F, Wang L, Duan X, Wang S (2018) Catalytic removal of aqueous contaminants on N-doped graphitic biochars: inherent roles of adsorption and nonradical mechanisms. Environ Sci Technol 52:8649–8658
Author information
Authors and Affiliations
Contributions
Conceptualization, formal analysis, visualization, validation and writing—original draft: Xiaowei Huo; conceptualization, validation, and writing—original draft: Chao Xue; formal analysis, visualization, and writing—original draft: Chenggui Zhang; data curation: Huichao Wang; conceptualization, formal analysis, visualization, and writing—original draft: Fuxiang Du; data curation: Chao Dai; writing—review and editing: Yang Yang; writing—review and editing: Cheng Lai; writing—review and editing: Junjun He. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, F., Huo, X., Xue, C. et al. Catalytic activation of persulfate by nanoscale zero-valent iron-derived supported boron-doped porous carbon for bisphenol A degradation. Environ Sci Pollut Res 31, 28241–28252 (2024). https://doi.org/10.1007/s11356-024-33035-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33035-0