Abstract
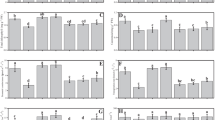
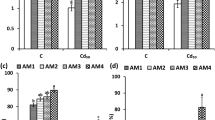
Chromium (Cr) contamination in soil–plant systems poses a pressing environmental challenge due to its detrimental impacts on plant growth and human health. Results exhibited that Cr stress decreased shoot biomass, root biomass, leaf relative water content, and plant height. However, single and co-application of Bacillus subtilis (BS) and arbuscular mycorrhizal fungi (AMF) considerably enhanced shoot biomass (+ 21%), root biomass (+ 2%), leaf relative water content (+ 26%), and plant height (+ 13) under Cr stress. The frequency of mycorrhizal (F) association (+ 5%), mycorrhizal colonization (+ 13%), and abundance of arbuscules (+ 5%) in the non-stressed soil was enhanced when inoculated with combined BS and AMF as compared to Cr-stressed soil. The co-inoculation with BS and AMF considerably enhanced total chlorophyll, carotenoids, and proline content in Cr-stressed plants. Cr-stressed plants resulted in attenuated response in SOD, POD, CAT, and GR activities when inoculated with BS and AMF consortia by altering oxidative stress biomarkers (H2O2 and MDA). In Cr-stressed plants, the combined application of BS and AMF considerably enhanced proline metabolism, for instance, P5CR (+ 17%), P5CS (+ 28%), OAT (− 22%), and ProDH (− 113%) as compared to control. Sole inoculation with AMF downregulated the expression of SIPIP2;1, SIPIP2;5, and SIPIP2;7 in Cr-stressed plants. However, the expression of NCED1 was downregulated with the application of sole AMF. In contrast, the relative expression of Le4 was upregulated in the presence of AMF and BS combination in Cr-stressed plants. Therefore, it is concluded that co-application of BS and AMF enhanced Cr tolerance by enhancing proline metabolism, antioxidant enzymes, and aquaporin gene expression. Future study might concentrate on elucidating the molecular processes behind the synergistic benefits of BS and AMF, as well as affirming their effectiveness in field experiments under a variety of environmental situations. Long-term research on the effect of microbial inoculation on soil health and plant production might also help to design sustainable chromium remediation solutions.







Similar content being viewed by others
Data Availability
The data will be avaliable upon request.
Change history
18 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11356-024-32965-z
References
Aebi Hugo (1984) Catalase in vitro. In Methods in enzymology. Academic press. 105:121–126
Ahmad H, Hayat S, Ali M, Liu T, Cheng Z (2018) The combination of arbuscular mycorrhizal fungi inoculation (Glomus versiforme) and 28‐homobrassinolide spraying intervals improves growth by enhancing photosynthesis, nutrient absorption, and antioxidant system in cucumber (Cucumis sativus L.) under salinity. Ecol Evol 8(11):5724–5740
Allah Abd EF, Alqarawi AA, Hashem A, Radhakrishnan R, Al-Huqail AA, Al-Otibi FON, Malik JA, Alharbi RI, Egamberdieva D (2018) Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J Plant Inter 13(1):37–44
Allen MF (2007) Mycorrhizal fungi: highways for water and nutrients in arid soils. Vadose Zone J 6(2):291–297
Apte AD, Verma S, Tare V, Bose P (2005) Oxidation of Cr (III) in tannery sludge to Cr (VI): field observations and theoretical assessment. J Hazard Mater 121(1–3):215–222
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris Plant Physiol 24(1):1
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11(1):3–42
Bárzana G, Aroca R, Bienert GP, Chaumont F, Ruiz-Lozano JM (2014) New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol Plant Microbe Interact 27(4):349–363
Branco S, Schauster A, Liao HL, Ruytinx J (2022) Mechanisms of stress tolerance and their effects on the ecology and evolution of mycorrhizal fungi. New Phytol 235(6):2158–2175
Calvo-Polanco M, Molina S, Zamarreño AM, García-Mina JM, Aroca R (2014) The symbiosis with the arbuscular mycorrhizal fungus Rhizophagus irregularis drives root water transport in flooded tomato plants. Plant Cell Physiol 55(5):1017–1029
Cheng HQ, Ding YE, Shu B, Zou YN, Wu QS, Kuča K (2020) Plant aquaporin responses to mycorrhizal symbiosis under abiotic stress. Intl J Agric Biol 23:786–794
Chilson OP, Kelly-Chilson AE, Siegel NR (1991) Pyrroline-5-carboxylate reductase in soybean nodules: isolation/partial primary structure/evidence for isozymes. Arch Biochem Biophys 288(2):350–357
Ding Y-E, Fan Q-F, He J-D, Wu H-H, Zou Y-N, Wu Q-S, Kuča K (2020) Effects of mycorrhizas on physiological performance and root TIPs expression in trifoliate orange under salt stress. Arch Agronom Soil Sci 66(2):182–192
Flores-Duarte NJ, Pajuelo E, Mateos-Naranjo E, Navarro-Torre S, Rodríguez-Llorente ID, Redondo-Gómez S, Carrasco López JA (2023) A culturomics-based bacterial synthetic community for improving resilience towards arsenic and heavy metals in the nutraceutical plant Mesembryanthemum crystallinum. Int J Mol Sci 24(8):7003
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9(4):436–442
Gadd GM (2004) Microbial influence on metal mobility and application for bioremediation. Geoderma 122(2–4):109–119
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in Higher Plants Plant Physiol 59(2):309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Harman G, Khadka R, Doni F, Uphoff N (2021) Benefits to plant health and productivity from enhancing plant microbial symbionts. Front Plant Sci 11:610065
Hashem A, Tabassum B, Allah Abd EF (2019) Bacillus subtilis: a plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J Biol Sci 26(6):1291–1297
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7(11):1456–1466
He F, Zhang H, Tang M (2016) Aquaporin gene expression and physiological responses of Robinia pseudoacacia L. to the mycorrhizal fungus Rhizophagus irregularis and drought stress. Mycorrhiza 26:311–323
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Hossain MA, Hoque MA, Burritt DJ, Fujita M (2014) Proline protects plants against abiotic oxidative stress: biochemical and molecular mechanisms. In Oxidative damage to plants (pp. 477–522). Elsevier
Hu B, Jia X, Hu J, Xu D, Xia F, Li Y (2017) Assessment of heavy metal pollution and health risks in the soil-plant-human system in the Yangtze River Delta, China. Int J Environ Res Public Health 14(9):1042
Jia-Dong H, Tao D, Hui-Hui W, Ying-Ning Z, Qiang-Sheng W, Kamil K (2019) Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci Hortic 243:64–69
Jiang C, Song X, He H, Chu L, Zhou H, Zhao Y, Xu Y, Zeng W, Lin X, Lu M-Z (2020) Genome-wide identification of plasma membrane aquaporin gene family in Populus and functional identification of PIP1;1 involved in osmotic stress. Environ Exp Bot 179:104200
Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Wigge PA (2016) Phytochromes function as thermosensors in Arabidopsis. Science, 354(6314):886–889
Kapilan R, Vaziri M, Zwiazek JJ (2018) Regulation of aquaporins in plants under stress. Biol Res 51(1):1–11
Kapoor R, Evelin H, Mathur P, Giri B (2012) Arbuscular mycorrhiza: approaches for abiotic stress tolerance in crop plants for sustainable agriculture. In Plant acclimation to environmental stress (pp. 359–401). Springer.
Karimi N, Ghasempour H-R (2019) Salicylic acid and jasmonic acid restrains nickel toxicity by ameliorating antioxidant defense system in shoots of metallicolous and non-metallicolous Alyssum inflatum Náyr. Populat Plant Physiol Biochem 135:450–459
Koza NA, Adedayo AA, Babalola OO, Kappo AP (2022) Microorganisms in plant growth and development: roles in abiotic stress tolerance and secondary metabolites secretion. Microorganisms 10(8):1528
Kumar A, Dames JF, Gupta A, Sharma S, Gilbert JA, Ahmad P (2015) Current developments in arbuscular mycorrhizal fungi research and its role in salinity stress alleviation: a biotechnological perspective. Crit Rev Biotechnol 35(4):461–474
Liang W-H, Li L, Zhang F, Liu Y-X, Li M-M, Shi H-H, Li H, Shang F, Lou C, Lin Q-T (2013) Effects of abiotic stress, light, phytochromes and phytohormones on the expression of OsAQP, a rice aquaporin gene. Plant Growth Regul 69(1):21–27
Lingua G, Bona E, Manassero P, Marsano F, Todeschini V, Cantamessa S, Copetta A, D’Agostino G, Gamalero E, Berta G (2013) Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria x ananassa var. Selva) in conditions of reduced fertilization. Intl J Mol Sci 14(8):16207–16225
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Maurel C, Verdoucq L, Luu D-T, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Moreira H, Pereira SI, Vega A, Castro PM, Marques AP (2020) Synergistic effects of arbuscular mycorrhizal fungi and plant growth-promoting bacteria benefit maize growth under increasing soil salinity. J Environ Manage 257:109982
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49(1):249–279
Pacioni G (1992) 16 wet-sieving and decanting techniques for the extraction of spores of vesicular-arbuscular fungi. Methods Microbiol 24:317–322
Porcel R, Barea JM, Ruiz-Lozano JM (2003) Antioxidant activities in mycorrhizal soybean plants under drought stress and their possible relationship to the process of nodule senescence. New Phytol 157(1):135–143
Quiroga G, Erice G, Ding L, Chaumont F, Aroca R, Ruiz-Lozano JM (2019) The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant, Cell Environ 42(7):2274–2290
Team R (2021) RStudio: integrated development for R. RStudio, PBC, Boston, MA. 2020
Reinhardt H, Hachez C, Bienert MD, Beebo A, Swarup K, Voß U, Bouhidel K, Frigerio L, Schjoerring JK, Bennett MJ (2016) Tonoplast aquaporins facilitate lateral root emergence. Plant Physiol 170(3):1640–1654
Riaz M, Kamran M, Fang Y, Wang Q, Cao H, Yang G, Deng L, Wang Y, Zhou Y, Anastopoulos I (2021) Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: a critical review. J Hazard Mater 402:123919
Ruiz-Lozano JM, Aroca R (2017) Plant aquaporins and mycorrhizae: their regulation and involvement in plant physiology and performance. Plant Aquaporins: From Transport to Signaling, 333–353
Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M (2021) Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants 10(2):277
Scoccianti V, Crinelli R, Tirillini B, Mancinelli V, Speranza A (2006) Uptake and toxicity of Cr(III) in celery seedlings. Chemosphere 64(10):1695–1703
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178:513–533
Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS, Jasrotia S, Zheng B, Yuan H, Yan D (2020) Chromium bioaccumulation and its impacts on plants: an overview. Plants 9(1):100
Shi Z, Zhang J, Wang F, Li K, Yuan W, Liu J (2018) Arbuscular mycorrhizal inoculation increases molybdenum accumulation but decreases molybdenum toxicity in maize plants grown in polluted soil. RSC Adv 8(65):37069–37076
Signorelli S, Tarkowski ŁP, O’Leary B, Tabares-da Rosa S, Borsani O, Monza J (2021) GABA and proline metabolism in response to stress. Hormones and Plant Response, 291–314.
Smith SE, Read DJ (2010) Mycorrhizal symbiosis. Academic press.
Tuteja N (2007) Abscisic acid and abiotic stress signaling. Plant Signal Behav 2(3):135–138
Ukhurebor KE, Aigbe UO, Onyancha RB, Nwankwo W, Osibote OA, Paumo HK, Siloko IU (2021) Effect of hexavalent chromium on the environment and removal techniques: a review. J Environ Manag 280:111809
Ulhassan Z, Khan I, Hussain M, Khan AR, Hamid Y, Hussain S, Zhou W (2022) Efficacy of metallic nanoparticles in attenuating the accumulation and toxicity of chromium in plants: Current knowledge and future perspectives. Environ Pollut, 120390
Vázquez MD, Poschenrieder C, Barcelo J (1987) Chromium VI induced structural and ultrastructural changes in bush bean plants (Phaseolus vulgaris L). Annals Botany 59(4):427–438
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64(12):5004–5007
Wadhwa N, Mathew BB, Jatawa S, Tiwari A (2012) Lipid peroxidation: mechanism, models and significance. Int J Curr Sci 3(1):29–38
Wu Q, Zou Y (2009) Mycorrhizal influence on nutrient uptake of citrus exposed to drought stress. Phil Agri Sci 92(1):33–38
Wu Q-S, Zou Y-N, He X-H (2010) Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol Plant 32:297–304
Wu Q-S, Srivastava AK, Zou Y-N (2013a) AMF-induced tolerance to drought stress in citrus: a review. Sci Hortic 164:77–87
Wu Q-S, Zou Y-N, Huang Y-M (2013b) The arbuscular mycorrhizal fungus Diversispora spurca ameliorates effects of waterlogging on growth, root system architecture and antioxidant enzyme activities of citrus seedlings. Fungal Ecol 6(1):37–43
Wu H-H, Zou Y-N, Rahman MM, Ni Q-D, Wu Q-S (2017) Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci Rep 7(1):42389
Wu Q-S, He J-D, Srivastava A, Zou Y-N, Kuča K (2019) Mycorrhizas enhance drought tolerance of citrus by altering root fatty acid compositions and their saturation levels. Tree Physiol 39(7):1149–1158
Xie M-M, Zou Y-N, Wu Q-S, Zhang Z-Z, Kuča K (2020) Single or dual inoculation of arbuscular mycorrhizal fungi and rhizobia regulates plant growth and nitrogen acquisition in white clover. Plant Soil Environ 66(6):287–294
Zhang F, Wang P, Zou Y-N, Wu Q-S, Kuča K (2019) Effects of mycorrhizal fungi on root-hair growth and hormone levels of taproot and lateral roots in trifoliate orange under drought stress. Arch Agro Soil Sci 65(9):1316–1330
Zou Y-N, Liang Y-C, Wu Q-S (2013a) Mycorrhizal and non-mycorrhizal responses to salt stress in trifoliate orange: plant growth, root architecture and soluble sugar accumulation. Int J Agric Biol 15(3):565–569
Zou Y-N, Wu Q-S, Huang Y-M, Ni Q-D, He X-H (2013b) Mycorrhizal-mediated lower proline accumulation in Poncirus trifoliata under water deficit derives from the integration of inhibition of proline synthesis with increase of proline degradation. PLoS ONE 8(11):e80568
Zou Y-N, Wu H-H, Giri B, Wu Q-S, Kuča K (2019) Mycorrhizal symbiosis down-regulates or does not change root aquaporin expression in trifoliate orange under drought stress. Plant Physiol Biochem 144:292–299
Zou Y-N, Zhang F, Srivastava AK, Wu Q-S, Kuča K (2021a) Arbuscular mycorrhizal fungi regulate polyamine homeostasis in roots of trifoliate orange for improved adaptation to soil moisture deficit stress. Front Plant Sci 11:600792
Zou YN, Wu QS, Kuča K (2021b) Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol 23:50–57
Zulfiqar U, Haider FU, Ahmad M, Hussain S, Maqsood MF, Ishfaq M, Eldin SM (2023) Chromium toxicity, speciation, and remediation strategies in soil-plant interface: a critical review. Front Plant Sci 13:1081624
Funding
This project was supported by Researchers Supporting Project number (RSP2024R230) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Tariq Shah conceived and designed the experiment; Tariq Shah prepared the materials and data collection; M. Asad assisted in software and statistical analysis of the data; Zeeshan Khan wrote the first draft of the manuscript; Ayesha Imran assisted in the software used for graphical representation of data; S. Khan assisted in materials and reagents preparation; Tahani AA. assisted in review of manuscript and funding; Mohammad Javed Ansari assisted in review, editing, and approval of final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, T., Khan, Z., Alahmadi, T.A. et al. Mycorrhizosphere bacteria inhibit chromium uptake and phytotoxicity by regulating proline metabolism, antioxidant defense system, and aquaporin gene expression in tomato. Environ Sci Pollut Res 31, 24836–24850 (2024). https://doi.org/10.1007/s11356-024-32755-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32755-7