Abstract
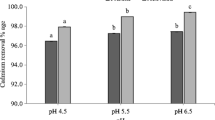
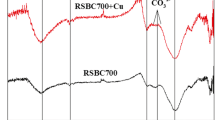
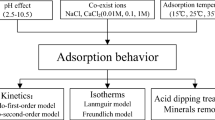
In recent years, cadmium pollution in water environment has become an environmental problem that could not be ignored. As a porous carbon rich solid material, biochar is an environment-friendly new material because of its ultra-high adsorption capacity and strong chemical stability. In this study, rice straw biochar (RS-Biochar) was successfully prepared at different temperatures for removal of Cd(II) from aqueous solution. Through a series of characterization and adsorption experiments, the adsorption principle of Cd(II) by RS-Biochar was deeply studied. The results showed that RS-Biochar prepared at 600 °C (BioC600) has high specific surface area (232.6 m2/g) and shows high Cd(II) removal rate of 91.23% with the maximum Cd(II) adsorption capacity of 8.62 mg/g. The Langmuir model fit well to describe the adsorption process of Cd(II) on the BioC600. The mechanism analysis showed that hydroxyl and carboxyl groups on the biochar surface were concerned in the removal of Cd(II). The formation of CdCO3 in the adsorption process was also be proven. Importantly, RS-Biochar could be conveniently produced with needed scale, displaying a promising approach for remediating Cd(II)-contaminated water environment and a huge application potential.








Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Ahmad Z, Gao B, Mosa A, Yu H, Yin X, Bashir A, Ghoveisi H, Wang S (2018) Removal of Cu (II), Cd (II) and Pb (II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J Clean Prod 180:437–449
Ahmed MJ, Hameed BH (2019) Insights into the isotherm and kinetic models for the coadsorption of pharmaceuticals in the absence and presence of metal ions: a review. J Environ Manag 252:109617
Bahadur V, Gadi R, Kalra S (2019) Clay based nanocomposites for removal of heavy metals from water: a review. J Environ Manage 232:803–817
Bandara T, Xu JM, Potter ID, Franks A, Chathurika JBAJ, Tang CX (2020) Mechanisms for the removal of Cd(II) and Cu(II) from aqueous solution and mine water by biochars derived from agricultural wastes. Chemosphere 254:126745.
Cha JS, Park SH, Jung SC, Ryu C, Jeon JK, Shin MC, Park YK (2016) Production and utilization of biochar: a review. J Ind Eng Chem 40:1–15
Chen S, Qin C, Wang T, Chen F, Li X, Hou H, Zhou M (2019) Study on the adsorption of dyestuffs with different properties by sludge-rice husk biochar: adsorption capacity, isotherm, kinetic, thermodynamics and mechanism. J Mol Liq 285:62–74
Chen YD, Huang MJ, Huang B, Chen XR (2012) Mesoporous activated carbon from inherently potassium-rich pokeweed by in situ self-activation and its use for phenol removal. J Anal Appl Pyrolysis 98:159–165
Conti R, Fabbri D, Vassura I, Ferroni L (2016) Comparison of chemical and physical indices of thermal stability of biochars from different biomass by analytical pyrolysis and thermogravimetry. J Anal Appl Pyrol 122:160–168
Cui X, Fang S, Yao Y, Li T, Ni Q, Yang X, He Z (2016) Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci Total Environ 562:517–525
Deng R, Huang DL, Wan J, Xue WJ, Lei L, Wen XF, Liu XG, Chen S, Yang Y, Li ZH, Li B (2019) Chloro-phosphate impregnated biochar prepared by co-precipitation for the lead, cadmium and copper synergic scavenging from aqueous solution. Bioresour Technol 293:122102
Domingues RR, Trugilho PF, Silva CA, De Melo ICNA, Melo LCA, Magriotis ZM, Sánchez-Monedero MA (2017) Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS One 12:0176884
Du L, Xu WH, Liu SB, Li X, Huang DL, Tan XF, Liu YG (2020) Activation of persulfate by graphitized biochar for sulfamethoxazole removal: The roles of graphitic carbon structure and carbonyl group. J Colloid Interf Sci 577:419–430
Fan SS, Fan XR, Wang S, Li B, Zhou N, Xu H (2023) Effect of chitosan modification on the properties of magnetic porous biochar and its adsorption performance towards tetracycline and Cu2+. Sustain Chem Pharm 33:101057
Gao LY, Deng JH, Huang GF, Li K, Cai KZ, Liu Y, Huang F (2019) Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour Technol 272:114–122
Gao ZY, Shan DX, He JH, Huang T, Mao Y, Tan HP, Shi HT, Li TZ, Xie TP (2023) Effects and mechanism on cadmium adsorption removal by CaCl2-modified biochar from selenium-rich straw. Bioresour Technol 370:128563
He H, Lu Q, Huang HH, Xue F, Lin WJ, Zhou H et al (2020) Biomass bagasse-based hyperbranched adsorbent for the complete removal of low-level Cr(VI). Cellulose 27:8121–8134
Huang DL, Wang Y, Zhang C, Zeng GM, Lai C, Wan J, Qin L, Zeng YL (2016) Influence of morphological and chemical features of biochar on hydrogen peroxide activation: implications on sulfamethazine degradation. RSC Adv 6(77):73186–73196
Huang F, Gao LY, Wu RR, Wang H, Xiao RB (2020) Qualitative and quantitative characterization of adsorption mechanisms for Cd2+ by silicon-rich biochar. Sci Total Environ 731:139163
Huang WH, Wu RM, Chang JS, Juang SY, Lee DJ (2023) Manganese ferrite modified agricultural waste-derived biochars for copper ions adsorption. Bioresour Technol 367:128303
Ippolito JA, Cui LQ, Kammann C, Wrage-Mönnig N, Estavillo JM, Fuertes-Mendizabal T, Cayuela ML, Sigua G, Novak J, Spokas K, Borchard N (2020) Feedstock choice, pyrolysis temperature and type influence biochar characteristics: a comprehensive meta-data analysis review. Biochar 2:421–438
Jaishankar M, Tseten T, Anbalagan N (2014) Toxicity, mechanism and health effects of some heavy metals. J Interdiscip Toxicol 7:60–72
Kalinke C, Mangrich AS, Marcolino LH, Bergamini MF (2016) Biochar prepared from castor oil cake at different temperatures: a voltammetric study applied for Pb2+, Cd2+ and Cu2+ ions preconcentration. J Hazard Mater 318:526–532
Ke YX, Zhang FX, Zhang ZL, Hough R, Fu Q, Li YF, Cui S (2023) Effect of combined aging treatment on biochar adsorption and speciation distribution for Cd (II). Sci Total Environ 867:161593
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass derived black carbon (biochar). Environ Sci Technol 44:1247–1253
Khurana P, Pulicharla R, Kaur Brar S (2021) Antibiotic-metal complexes in wastewaters: fate and treatment trajectory. Environ Int 157:106863
Kim HB, Kim JG, Kim T, Alessi DS, Baek K (2021) Interaction of biochar stability and abiotic aging: influences of pyrolysis reaction medium and temperature. Chem Eng J 411:128411
Kong X, Gao H, Song X, Deng Y, Zhang Y (2020) Adsorption of phenol on porous carbon from Toona sinensis leaves and its mechanism. Chem Phys Lett 739:137046
Kubier A, Hamer K, Pichler T (2020) Cadmium background levels in groundwater in an area dominated by agriculture. Integr Environ Assess Manag 16:103–113
Li XY, Ai LH, Jiang J (2016) Nanoscale zero valent iron decorated on graphene nanosheets for Cr (VI) removal from aqueous solution: surface corrosion retard induced the enhanced performance. Chem Eng J 288:789–797
Liu PY, Rao DA, Zou LY, Teng Y, Yu HY (2021) Capacity and potential mechanisms of Cd (II) adsorption from aqueous solution by blue algae-derived biochars. Sci Total Environ 767:145447
Liu XQ, Ding HS, Wang YY, Liu WJ, Jiang H (2016) Pyrolytic temperature dependent and ash catalyzed formation of sludge char with ultra-high adsorption to 1-Naphthol. Environ Sci Technol 50:2602–2609
Lun MK, Lin H, He YH, Li B, Dong YB, Wang L (2019) Efficient simultaneous removal of cadmium and arsenic in aqueous solution by titanium-modified ultrasonic biochar. Bioresour Technol 284:333–339
Luo JW, Li X, Ge CJ, Muller K, Yu HM, Huang P et al (2018) Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems. Bioresour Technol 263:385–392
Mahajan M, Gupta PK, Singh A, Vaish B, Singh P, Kothari R, Singh RP (2021) A comprehensive study on aquatic chemistry health risk and remediation techniques of cadmium in groundwater. Sci Total Environ 818:151784
Meng J, Wang LL, Liu XM, Wu JJ, Brookes PC, Xu JM (2013) Physicochemical properties of biochar produced from aerobically composted swine manure and its potential use as an environmental amendment. Bioresour Technol 142:641–646
Minh Loy AC, Yusup S, Fui Chin BL, Wai Gan DK, Shahbaz M, Acda MN, Unrean P, Rianawati E (2018) Comparative study of in-situ catalytic pyrolysis of rice husk for syngas production: kinetics modelling and product gas analysis. J Clean Prod 197:1231–1243
Novak LI, Xing B, Gaskin JW, Steiner C, Das KC, Ahmedna M, Rehrah D, Watts DW, Busscher WJ, Schomberg H (2009) Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann Environ Sci 3(1):195–206
Park JH, Wang JJ, Xiao R, Wang M, Lee YH, Kang SW, Seo DC (2022) Characteristics of adsorption behavior of potentially toxic metals by biochar derived from fallen leaves (Platanus) and its mechanism. Sustain Chem Pharm 29:10077
Peng HB, Gao P, Chu G, Pan B, Peng JH, Xing BS (2017) Enhanced adsorption of Cu (II) and Cd (II) by phosphoric acid-modified biochars. Environ Pollut 229:846–853
Qambrani NA, Rahman MM, Won S, Shim S, Ra C (2017) Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: a review. Renew Sustain Energy Rev 79:255–273
Saeed AAH, Harun NY, Sufian S, Bilad MR, Zakaria ZY, Jagaba AH, Ghaleb AAS, Mohammed HG (2021) Pristine and magnetic kenaf fiber biochar for Cd2+ adsorption from aqueous solution. Int J Environ Res Public Health 18:7949
Shakya A, Vithanage M, Agarwal T (2022) Influence of pyrolysis temperature on biochar properties and Cr (VI) adsorption from water with groundnut shell biochars: Mechanistic approach. Environ Res 215:114243
Shi Y, Shan R, Lu L, Yuan H, Jiang H, Zhang Y, Chen Y (2020) High-efficiency removal of Cr (VI) by modified biochar derived from glue residue. J Clean Prod 254:119935
Shin W (2017) Adsorption characteristics of phenol and heavy metals on biochar from Hizikia fusiformis. Environ Earth Sci 76:1–9
Singh E, Kumar A, Mishra R et al (2021) Pyrolysis of waste biomass and plastics for production of biochar and its use for removal of heavy metals from aqueous solution. Bioresour Technol 320:124278
Spokas KA (2010) Review of the stability of biochar in soils: predictability of O: C molar ratios. Carbon Manag 1:289–303
Teng D, Zhang B, Xu G, Wang B, Mao K, Wang J, Sun J, Feng X, Yang Z, Zhang H (2020) Efficient removal of Cd(II) from aqueous solution by pinecone biochar: sorption performance and governing mechanisms. Environ Pollut 265:115001
Tian S, Zhang C, Huang D, Wang R, Zeng G, Yan M, Xiong W, Zhou C, Cheng M, Xue W, Yang Y, Wang W (2020) Recent progress in sustainable technologies for adsorptive and reactive removal of sulfonamides. Chem Eng J 389:123423
Vassilev SV, Vassileva CG (2016) Composition, properties and challenges of algae biomass for biofuel application: an overview. Fuel 181:1–33
Wang H, Huang F, Zhao ZL, Wu RR, Xu WX, Wang P, Xiao RB (2021) High efficiency removal capacities and quantitative adsorption mechanisms of Cd2+ by thermally modified biochars derived from different feedstocks. Chemosphere 272:129594
Wang XD, Li CX, Li ZW, Yu GW, Wang X (2019) Effect of pyrolysis temperature on characteristics, chemical speciation and risk evaluation of heavy metals in biochar derived from textile dyeing sludge. Ecotox Environ Safe 168:45–52
Wang XY, Zhao YW, Deng J, Zhou YJ, Yuan SF (2023) Study on the influence mechanism of mineral components in biochar on the adsorption of Cr(VI). Fuel 340:127631
Weber K, Quicker P (2018) Properties of biochar. Fuel 217:240–261
Wu J, Wang T, Wang J, Zhang Y, Pan WP (2021) A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: Enhanced the ion exchange and precipitation capacity. Sci Total Environ 754:142150
Yin W, Zhao C, Xu J (2019) Enhanced adsorption of Cd (II) from aqueous solution by a shrimp bran modified Typha orientalis biochar. Environ Sci Pollut Res Int 26(36):37092–37100
Yu XN, Zhou HJ, Ye XF, Wang HL (2021) From hazardous agriculture waste to hazardous metal scavenger: tobacco stalk biochar-mediated sequestration of Cd leads to enhanced tobacco productivity. J Hazard Mater 413:125303
Yuan P, Wang J, Pan Y, Shen B, Wu C (2019) Review of biochar for the management of contaminated soil: preparation, application and prospect. Sci Total Environ 659:473–490
Yuan QS, Wang PF, Wang X, Hu B, Wang C, Xing XL (2023) Nano-chlorapatite modification enhancing cadmium(II) adsorption capacity of crop residue biochars. Sci Total Environ 865:161097
Zhang S, Dong Q, Zhang L, Xiong Y, Liu X, Zhu S (2015) Effects of water washing and torrefaction pretreatments on rice husk pyrolysis by microwave heating. Bioresour Technol 193:442–448
Zhou Y, Chen Z, Gong H, Chen L, Yu H, Wu W (2019) Characteristics of dehydration during rice husk pyrolysis and catalytic mechanism of dehydration reaction with NiO/g-Al2O3 as catalyst. Fuel 245:131–138
Funding
This work was supported by the National Natural Science Foundation of China (51908233) and National Natural Science Foundation of China (41907124).
Author information
Authors and Affiliations
Contributions
Qiao Xiong: methodology, formal analysis, software, investigation, writing — original draft. Yinqiu Li: investigation. Chaohua Hou: investigation. Xiao Ma: software, validation. Xiangjun Zhou: software, validation. Xiangru Zuo: supervision. Chang Chen: writing — review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiong, Q., Li, Y., Hou, C. et al. An efficient and simple approach to remove Cd(II) in aqueous solution by using rice straw biochar: performance and mechanisms. Environ Sci Pollut Res 31, 16782–16794 (2024). https://doi.org/10.1007/s11356-024-32222-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32222-3