Abstract
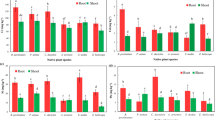
Cobalt (Co) is considered an essential element in agriculture as it is an important constituent of vitamin B12. Due to natural and anthropogenic factors, heavy metals, especially Co, accumulate in agricultural fields, but their high exposure produces ramifications in crop plants, thereby reducing crop yield and biomass. Excessive Co in plants causes oxidative stress, and as the stress progresses, Co competes with iron (Fe) thereby decreasing chlorophyll content and resulting in Fe deficiency in plants. A major concern is to counter the Co toxicity. Therefore, the current study aimed to mitigate Co-stress or Co-toxicity by using siderophore producing microbes and simultaneously mobilize Co and iron (Fe) in required amounts. In this study, 250 bacteria were isolated from agricultural and non-agricultural soils and screened for siderophore production. Initial siderophore screening revealed that 28.8% of the isolates produced siderophore. Subsequent screening for Co-tolerance showed that 16 isolates were tolerant to up to 20,000 ppm of Co and produced ACC deaminase, siderophore (96.82–99.67%), indole-3-acetic acid (15.15–70.55 µg/mL) and phosphate solubilisation (39.33–142.67 µg/mL). A plate assay (200 mM Co stress) revealed that four isolates (KSBTS 12, SBTS 12, CWTS 5 and CWTS 10) enhanced the growth of black gram (Vigna mungo L.). Furthermore, evaluation in pot studies (2000 ppm Co stress) revealed enhanced root (60.69–174.24%) and shoot length (3.27–143.96%) compared to the control. Inoculated plants also enhanced the uptake of nitrogen (37.33–42.36 mg/g) and phosphorous (3.12–3.92 mg/g), chlorophyll content (7.60–22.97 mg/g), siderophore quantity in the soils (282.41–331.53%) and the soil respiration activity such as hydrolysis of fluorescein diacetate (11.33–24.88 µg/g), dehydrogenase enzyme (109.76–197.26 µg/g) and alkaline phosphatase (631.53–918.20 µg/g). In conclusion, CWTS 5 (Bacillus subtilis) and CWTS 10 (Bacillus albus) can be used to mitigate Co-stress and mobilize Co and Fe in plants.






Similar content being viewed by others
Data availability
On request, the data presented in this study is available from the authors.
References
Abbas MN, Al-Tameemi IM, Hasan MB, Al-Madhhachi AS (2021) Chemical removal of cobalt and lithium in contaminated soils using promoted white eggshells with different catalysts. S Afr J Chem Eng 35:23–32
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33:943–951
Ahmad N, Yasin D, Bano F, Fatma T (2022) Ameliorative effects of endogenous and exogenous indole-3-acetic acid on atrazine stressed paddy field cyanobacterial biofertilizer Cylindrospermum stagnale. Sci Rep 12:11175
Akeel A, Jahan A (2020) Role of cobalt in plants: its stress and alleviation. In: Contaminants in Agriculture: Sources, Impacts and Management. Springer, Cham, pp.339–57
Arnon DI, Stout PR (1939) The essentiality of certain elements in minute quantity for plants with special reference to copper. Plant Physiol 14:371–375
Babu AG, Kim JD, Oh BT (2013) Enhancement of heavy metal phytoremediation by Alnus firma with endophytic Bacillus thuringiensis GDB1. J Hazard Mater 250:477–483
Bessey OA, Lowky OH, Brock MJ (1946) A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J Biol Chem 164:321–329
Bouguerra S, Gavina A, Natal-da-Luz T, Sousa JP, Ksibi M, Pereira R (2022) The use of soil enzymes activity, microbial biomass, and basal respiration to assess the effects of cobalt oxide nanomaterial in soil microbiota. Appl Soil Ecol 169:104246
Casida LE Jr, Klein DA, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376
Chandwani S, Amaresan N (2022) Role of ACC deaminase producing bacteria for abiotic stress management and sustainable agriculture production. Environ Sci Pollut Res 29:22843–22859
Chandwani S, Chavan SM, Paul D, Amaresan N (2022) Bacterial inoculations mitigate different forms of iron (Fe) stress and enhance nutrient uptake in rice seedlings (Oryza sativa L.). Environ Technol Innov 26:102326
Da Silva MP, Pereira A, Rupe JC, Bluhm BH, Gbur EE, Wood L, Chen P (2019) Effectiveness of a seed plate assay for evaluating charcoal rot resistance in soybean and the relationship to field performance. Plant Dis 103:1947–1953
Das S, Barooah M (2017) Identification and characterization of siderophore producing arsenic tolerant Staphylococcus sp. TA6 and its possible involvement in arsenic geocycle. BioRxiv 231241.
Devi R, Kaur T, Kour D, Hricovec M, Mohan R, Yadav N, Yadav AN (2022) Microbes-mediated alleviation of heavy metal stress in crops: current research and future challenges. J Appl Biol Biotechnol 10:25–37
Efe D (2020) Potential plant growth-promoting bacteria with heavy metal resistance. Curr Microbiol 77:3861–3868
Etesami H (2018) Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: mechanisms and future prospects. Ecotoxicol Environ Saf 147:175–191
Freitas H, Prasad MNV, Pratas J (2004) Heavy metals in the plant community of Sao Domingos, an abandoned mine in SE Portugal: possible applications in mine remediation. Environ Int 30:65–72
Gang S, Sharma S, Saraf M, Buck M, Schumacher J (2019) Analysis of indole-3-acetic-acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. Bio Protoc 9:1–9
Glick BR, Patten CL, Holguin G, Penrose DM (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. World Scientific 1–13.
Gonzalez Henao S, Ghneim-Herrera T (2021) Heavy metals in soils and the remediation potential of bacteria associated with the plant microbiome. Front Environ Sci 9:604216
Gopal R, Dube BK, Sinha P, Chatterjee C (2003) Cobalt toxicity effects on growth and metabolism of tomato. Commun Soil Sci Plant Anal 34:619–628
Gopi K, Jinal HN, Prittesh P, Kartik VP, Amaresan N (2020) Effect of copper-resistant Stenotrophomonas maltophilia on maize (Zea mays) growth, physiological properties, and copper accumulation: potential for phytoremediation into biofortification. Int J Phytoremediation 22:662–668
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Hu X, Wei X, Ling J, Chen J (2021) Cobalt: an essential micronutrient for plant growth? Front Plant Sci 12:768523
Jinal HN, Gopi K, Prittesh P, Kartik VP, Amaresan N (2019) Phytoextraction of iron from contaminated soils by inoculation of iron-tolerant plant growth-promoting bacteria in Brassica juncea L. Czern Environ Sci Pollut Res 26:32815–32823
Jinal HN, Gopi K, Kumar K, Amaresan N (2020) Effect of zinc-resistant Lysinibacillus species inoculation on growth, physiological properties, and zinc uptake in maize (Zea mays L.). Environ Sci Pollut Res 28:6540–6548
Jones Jr JB (1991) Kjeldahl method for nitrogen determination. Micro-Macro Publishing, Inc., Athens, Georgia, p.7
Kartik VP, Jinal HN, Amaresan N (2021) Inoculation of cucumber (Cucumis sativus L.) seedlings with salt-tolerant plant growth promoting bacteria improves nutrient uptake, plant attributes and physiological profiles. J PlantGrowth Regul 40:1728–1740
Kroh GE, Pilon M (2020) Regulation of iron homeostasis and use in chloroplasts. Int J Mol Sci 21:3395
Kuffner M, De Maria S, Puschenreiter M, Fallmann K, Wieshammer G, Gorfer M, Sessitsch A (2010) Culturable bacteria from Zn-and Cd accumulating Salix caprea with differential effects on plant growth and heavy metal availability. J Appl Microbiol 108:1471–1484
Lewis RW, Islam A, Opdahl L, Davenport JR, Sullivan TS (2019) Comparative genomics, siderophore production, and iron scavenging potential of root zone soil bacteria isolated from ‘Concord’grape vineyards. Microb Ecol 78:699–713
Lurthy T, Cantat C, Jeudy C, Declerck P, Gallardo K, Barraud C, Leroy F, Ourry A, Lemanceau P, Salon C, Mazurier S (2020) Impact of bacterial siderophores on iron status and ionome in pea. Front Plant Sci 11:730
Lyu S, Wei X, Chen J, Wang C, Wang X, Pan D (2017) Titanium as a beneficial element for crop production. Front Plant Sci 8:597
Mahey S, Kumar R, Sharma M, Kumar V, Bhardwaj R (2020) A critical review on toxicity of cobalt and its bioremediation strategies. SN Appl Sci 2:1–2
Maslin P, Maier RM (2000) Rhamnolipid-enhanced mineralization of phenanthrene in organic-metal co-contaminated soils. Bioremediat J 4:295–308
Mustapha MU, Halimoon N (2015) Screening and isolation of heavy metal tolerant bacteria in industrial effluent. Procedia Environ Sci 30:33–37
Odaka M, Kobayashi M (2013) Cobalt proteins, overview. In: Kretsinger RH, Uversky VN, Permyakov EA (eds) Encyclopedia of Metalloproteins. Springer, New York, pp 670–678
Omar SA, Fetyan NA, Eldenary ME, Abdelfattah MH, Abd-Elhalim HM, Wrobel J, Kalaji HM (2021) Alteration in expression level of some growth and stress-related genes after rhizobacteria inoculation to alleviate drought tolerance in sensitive rice genotype. Chem Biol Technol Agric 8:1–19
Pais I (1992) Criteria of essentiality, beneficiality and toxicity of chemical elements. Acta Aliment 21:145–152
Paul D, Sinha SN (2017) Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river Ganga, India. Ann Agrar Sci 15:130–136
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Shaver LA (2008) Determination of phosphates by the gravimetric quimociac technique. J Chem Educ 85:1097
Shreya D, Jinal HN, Kartik VP, Amaresan N (2020) Amelioration effect of chromium-tolerant bacteria on growth, physiological properties and chromium mobilization in chickpea (Cicer arietinum) under chromium stress. Arch Microbiol 202:887–894
Soomro GA, Shar GA (2014) A simple spectrophotometric method for the determination of cobalt (II) using 1-nitroso-2-naphthol in anionic aqueous solution of sodium dodecyl sulphate. Int J Chem Sci 12:982–992
Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA (1996) Methods of soil analysis: part 3. Chemical Methods. Soil Science Society of America, Madison (WI), p. 20
Sree KS, Keresztes Á, Mueller-Roeber B, Brandt R, Eberius M, Fischer W, Appenroth KJ (2015) Phytotoxicity of cobalt ions on the duckweed Lemna minor–morphology, ion uptake, and starch accumulation. Chemosphere 131:149–156
Sritongon N, Sarin P, Theerakulpisut P, Riddech N (2022) The effect of salinity on soil chemical characteristics, enzyme activity and bacterial community composition in rice rhizospheres in Northeastern Thailand. Sci Rep 12:20360
Wa Lwalaba JL, Zvogbo G, Mulembo M, Mundende M, Zhang G (2017) The effect of cobalt stress on growth and physiological traits and its association with cobalt accumulation in barley genotypes differing in cobalt tolerance. J Plant Nutr 40:2192–2199
Acknowledgements
The authors thank Navsari Agricultural University (NAU) for analysing the physicochemical parameters of the soil samples. The authors would also like to thank the Soil Testing Laboratory, Dang, Gujarat, for analysing N, P, Fe and Co from the plant samples.
Funding
This work was supported by a Ph.D. fellowship granted by the Scheme of Developing High Quality Research (SHODH) of the Knowledge Consortium of Gujarat to SC.
Author information
Authors and Affiliations
Contributions
NA: designed the study and edited final manuscript; SC: performed the experiments, data analysis and drafting initial manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Additional information
Responsible Editor: Gangrong Shi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandwani, S., Amaresan, N. Siderophore-producing bacteria mitigate cobalt stress in black gram (Vigna mungo L.), and the mitigation strategies are associated with iron concentration. Environ Sci Pollut Res 30, 123556–123569 (2023). https://doi.org/10.1007/s11356-023-31106-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31106-2