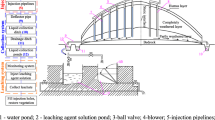
Abstract
The ion-exchangeable ammonium (IE-A) that accounts for 60–90% of the total residual ammonium in rare earth tailings has great potential to pollute the surrounding environment, and much research has been done to seek an effective elution method. However, the current study mainly focused on the single salt solution, which made it hard to reach the desired elution efficiency. In this study, the efficient binary compound eluent was prepared, and the response surface experiments and dynamic elution were performed to optimize the elution condition and evaluate the practical application prospect. Batch experimental results showed that the best IE-A elution efficiency could be achieved at the K:Mg molar ratio of 8:2, the liquid–solid ratio of 26:1, and the concentration of 0.1 mol/L at the natural solution pH. Dynamic experimental results indicated that a higher concentration, flow rate, and elution temperature could all accelerate the elution process, and the highest elution efficiency could reach 99%. The fitting results by shrinking core models show that the apparent activation energy of IE-A was 4.24 kJ/mol in the temperature range of 288–328 K, and the reaction order was 0.16. XPS and FTIR revealed that IE-A was effectively eluted by a potassium and magnesium compound leaching agent via an ion-exchange reaction. Overall, the developed compound solution with potassium and magnesium is a candidate for an elution agent that could be used to remove residual ammonium in a closed field of rare earth ores.
Graphical Abstract












Similar content being viewed by others
Data availability
Not applicable.
References
Aydogan S, Ucar G, Canbazoglu M (2006) Dissolution kinetics of chalcopyrite in acidic potassium dichromate solution. J Hydrometallurgy 81(1):45–51
Chai XW, Li GQ, Zhang ZY et al (2020) Leaching kinetics of weathered crust elution-deposited rare earth ore with compound ammonium carboxylate. J Minerals 10(6):516
Chi R, Liu X (2019) Prospect and development of weathered crust elution-deposited rare earth ore. J Rare Earths 37(2):129–140
Chi RA, Tian J (2006) Chemical Metallurgy of Weathered crust elution-deposited rare earth ores. Science Press, Beijing
Chi RA, Tian J (2008) Weathered crust elution-deposited rare earth ores. Nova Science Pub Inc., China
Ecer U, Sahan T (2018) A response surface approach for optimization of Pb (II) biosorption conditions from the aqueous environment with Polyporus squamosus fungi as a new biosorbent and kinetic, equilibrium and thermodynamic studies. J Desalin Water Treat 102:229–240
Fan X, Xue Q, Liu SW et al (2021) The influence of soil particle size distribution and clay minerals on ammonium nitrogen in weathered crust elution-deposited rare earth tailing. J Ecotox Environ Safe 208:111663
Feng J, Yu JX, Huang SX et al (2021) Effect of potassium chloride on leaching process of residual ammonium from weathered crust elution-deposited rare earth ore tailings. J Miner Eng 163:8
Guo LY, Chen QK, Fang F et al (2013) Application potential of a newly isolated indigenous aerobic denitrifier for nitrate and ammonium removal of eutrophic lake water. J Bioresour Technol 142:45–51
He ZY, Zhang ZY, Chi RA et al (2017) Leaching hydrodynamics of weathered elution-deposited rare earth ore with ammonium salts solution. J Rare Earths 35(8):824–830
Huang SX, Feng J, Yu JX et al (2021) Adsorption and desorption performances of ammonium on the weathered crust elution-deposited rare earth ore. J Colloid Surf A-Physicochem Eng Asp 613:10
Huang SX, Feng J, Ouyang Z et al (2022) Dynamic elution of residual ammonium leaching agent from weathered crust elution-deposited rare earth tailings by magnesium chloride. J Environ Res 210:8
Ji B, Zhang WC (2021) Rare earth elements (REEs) recovery and porous silica preparation from kaolinite. J Powder Technol 391:522–531
Katyal JC, Carter MF, Vlek PLG (1988) Nitrification activity in submerged soils and its relation to denitrification loss. J Biol Fertil Soils 7(1):16–22
Krakat N, Demirel B, Anjum R et al (2017) Methods of ammonia removal in anaerobic digestion: a review. J Water Sci Technol 76(8):1925–1938
Kynicky J, Smith MP, Xu C (2012) Diversity of rare earth deposits: the key example of China. J Elements 8(5):361–367
Levenspiel O (1972) Applied kinetics. (book reviews: chemical reaction engineering. an introduction to the design of chemical reactors). Science
Li XY, Min XB, Hu XX et al (2021) In-situ synthesis of highly dispersed Cu-CuxO nanoparticles on porous carbon for the enhanced persulfate activation for phenol degradation. J Sep Purif Technol 276:119260
Li YT, Tu AB, Zhang YF, Zhang M, Chi RA (2009) Kinetics of leaching rare earth from a weathered crust elution-deposited rare earth ore in South China with mixed ammonium salt. J Industrial Minerals and Processing 38(02):19–24
Liu YH, Chen J, Li DQ (2011) Application and perspective of ionic liquids on rare earths green separation. J Sep Sci Technol 47(2):223–232
Long P, Wang GS, Zhang C et al (2020) Kinetics model for leaching of ion-adsorption type rare earth ores. J Rare Earths 38(12):1354–1360
Lumbanraja J, Evangelou VP (1994) Adsorption-desorption of potassium and ammonium at low cation concentrations in three Kentucky subsoils. Soil Sci 157(5):269–278
Mashaba PM, Abdulsalam J, Bada SO (2023) Application of the response surface methodology tooptimize the characteristics of weathered discard coals using the w/o hip emulsion technique. Int J Coal Prep Util 43(3):468–483
Murray HH (2006) Chapter 2 structure and composition of the clay minerals and their physical and chemical properties. Developments in Clay Science 2:7–31
Nie WR, Zhang R, He ZY et al (2020) Research progress on leaching technology and theory of weathered crust elution-deposited rare earth. J Hydrometallurgy 193:105295
Nightingale E Jr (1959) Phenomenological theory of ion solvation. Effective radii of hydrated ions. J J Phys Chem 63(9):1381–1387
Qin L, Hu SL, Song CX et al (2021) Ammonia nitrogen leaching removal from ionic rare earth tailings. J Chin J Nonferrous Metals 31:1395–1403
Qiu TS, Zhu DM, Fang XH et al (2014) Leaching kinetics of ionic rare-earth in ammonia-nitrogen wastewater system added with impurity inhibitors. J Rare Earths 32(12):1175–1183
Schotting RJ, Moser H, Hassanizadeh SM (1999) High-concentration-gradient dispersion in porous media: experiments, analysis, and approximations. J Adv Water Resour 22(7):665–680
Sen K, Mondal NK, Chattoraj S et al (2017) Statistical optimization study of adsorption parameters for the removal of glyphosate on forest soil using the response surface methodology. J Environ Earth Sci 76(1):15
Tang J, Qiao JY, Xue Q et al (2018) Leach of the weathering crust elution-deposited rare earth ore for low environmental pollution with a combination of (NH4)2SO4 and EDTA. J Chemosphere 199:160–167
Teimouri S, Mawire G, Potgieter JH, Simate GS, Dyk LV, Dworzanowski M (2020) Using experimental design and response surface methodology (rsm) to optimize gold extraction from refractory sulphidic gold tailings with ionic liquids. J South Afr Inst Min Metall (7)
Tian J, Chi RA, Yin JQ (2010) Leaching process of rare earths from weathered crust elution-deposited rare earth ore. J Trans Nonferrous Met Soc China 20(5):892–896
Tian J, Tang XK, Yin JQ et al (2013) Process optimization on leaching of a lean weathered crust elution-deposited rare earth ores. J Int J Miner Process 119:83–88
Wan J, Zhao F, Meng Y et al (2021) Three-dimensional electrochemical degradation of p-aminophenol with efficient honeycomb block AC@Ti-Cu-Ni-Zn-Sb-Mn particle electrodes. J Sep Purif Technol 267:118662
Wang XG, Lu SY, Gao CM et al (2014) Highly efficient adsorption of ammonium onto palygorskite nanocomposite and evaluation of its recovery as a multifunctional slow-release fertilizer. J Chem Eng J 252:404–414
Zhang ZY, He ZY, Yu JX et al (2016) Novel solution injection technology for in-situ leaching of weathered crust elution-deposited rare earth ores. J Hydrometallurgy 164:248–256
Zhang QY, Ren FT, Li FD et al (2020) Ammonia nitrogen sources and pollution along soil profiles in an in-situ leaching rare earth ore. J Environ Pollut 267:115449
Zhou F, Liu Q, Feng J et al (2019) Role of initial moisture content on the leaching process of weathered crust elution-deposited rare earth ores. J Sep Purif Technol 217:24–30
Acknowledgements
The work is funded by National Natural Science Foundation of China (No. 52274267), National Key Research and Development Project (No. 2018YFC1801801), the Innovative Team program of Natural Science Foundation of Hubei Province (No. 2021CFA032), the Application foundation project of Wuhan Science and Technology Bureau (No. 2020020601012276), and the Program for Excellent Young Scientific and Technological Innovation Team of Hubei Provincial Department of Education, China (No. T201506).
Funding
The work is funded by the National Natural Science Foundation of China (No. 21978226), the National Key Research and Development Project (No. 2018YFC1801801), the Innovative Team program of Natural Science Foundation of Hubei Province (No. 2021CFA032), the Application Foundation Project of Wuhan Science and Technology Bureau (No. 2020020601012276), and the Program for Excellent Young Scientific and Technological Innovation Team of Hubei Provincial Department of Education, China (No. T201506), National project (No. 52274267).
Author information
Authors and Affiliations
Contributions
Xiaoju Li: conceptualization; methodology; investigation; formal analysis; writing—original draft; writing—review and editing. Junxia Yu: conceptualization, formal analysis, resources, writing—review and editing. Xiaodi Li: editing, investigation, methodology, resources. Guoping Song: resources. Ze Ouyang: formal analysis. Rong Wang: formal analysis. Zhenyue Zhang: methodology, validation, resources, supervision. Chunqiao Xiao: investigation. Ruan Chi: methodology; validation; formal analysis; resources; project administration; writing—review and editing; supervision; funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Yu, J., Li, X. et al. Synergistic leaching process for ion-exchange ammonium from weathered crust elution deposited rare earth tailings with potassium magnesium compound eluent. Environ Sci Pollut Res 30, 121513–121528 (2023). https://doi.org/10.1007/s11356-023-30879-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30879-w