Abstract
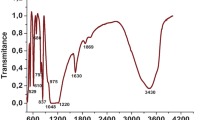
Selective and rapid determination of procedure for Cd2+ and Pb2+ samples using MWCNT surfaces can be modified by loading ligands such as D2EHPA and Cyanex 272 which is described. The adsorbent was modified with D2EHPA and Cyanex 272. Effect of pH, amount of adsorbent, contact time for adsorption, and the optimum eluent for the quantitative recovery of Cd2+ and Pb2+ were investigated and the subsequent determination by FAAS. The adsorption was found to be mainly due to the chemical interactions between the metal ions and functional groups –COO− and –OH which were characterized by FT-IR. The adsorption of metal occurs at pH 4.5 with 500 mg of MWCNTs. The enrichment factor was 40 and 30. The detection limit was 0.03 and 0.05 μg L−1. The quantitative recovery of metal ion used 1 mol L−1 HNO3. The thermodynamic parameter of Langmuir and Freundlich adsorption isotherm revealed that the adsorption of free energy (ΔG) was spontaneous and the monolayer adsorption of Cd2+ and Pb2+ was mainly on the surfaces. The adsorbent performance of R2 in the range of 0.93–0.99 and also the identified adsorption efficiency of Cd2+ and Pb2+ are linear or non-linear curves respectively. The proposed method was applied to heavy metals from environmental samples.
Graphical Abstract







Similar content being viewed by others
References
Aziz MA, Chowdhury IR, Mazumder MAJ (2019) Highly porous carboxylated activated carbon from jute stick for removal of Pb2+ from aqueous solution. Environ Sci Pollut Res 26:22656–22669
Barbosa A, Segatelli M, Pereira A, Santos A, Kubota L, Luccas P, Tarley C (2007) Solid-phase extraction system for Pb (II) ions enrichment based on multiwall carbon nanotubes coupled on-line to flame atomic absorption spectrometry. Talanta 71(40):1512–1519
Barrera PB, Nancy MA, Cristina DL, Adela BB (2003) Use of Amberlite XAD-2 loaded with 1-(2- pyridylazo)-2-naphthol as a preconcentration system for river water prior to determination of Cu2+ , Cd2+ and Pb2+ by flame atomic absorption spectroscopy. Microchim Acta 142(1):101–108
Baytak S, Turker AR (2006) Determination of lead and nickel in environmental samples by flame atomic absorption spectrometry after column solid-phase extraction on Ambersorb-572 with EDTA. J Hazard Mater B129(1-3):130–136
Benamor M, Bouariche Z, Belaid T, Draa MT (2008) Kinetic studies on cadmium ions by Amberlite XAD7 impregnated resins containing di(2-ethylhexyl) phosphoric acid as extractant. Sep Purif Technol 59(1):74–84
Chen S, Liu C, Yang M, Lu D, Zhu LJ (2009) Solid-phase extraction of Cu, Co and Pb on oxidized single-walled carbon nanotubes and their determination by inductively coupled plasma mass spectrometry. J Hazard Mater 70:247
Del Rio M, Font R, Almela C, Velez D, Montoro R, Bailon ADH (2002) Heavy metals and arsenic uptake by wild vegetation in the Guadiamar river area after the toxic spill of the Aznalcollar mine. J Biotechnol 98(2):125–137
Demirata B, Tor I, Filik H, Afsar H (1996) Separation of Cr(III) and Cr(VI) using melamine-formaldehyde resin and determination of both species in water by FAAS. Fresenius J Anal Chem 356(2):375–377
El-Sheikh AH (2008) Effect of oxidation of activated carbon on its enrichment efficiency of metal ions: comparison with oxidized and non-oxidized multi-walled carbon nanotubes. Talanta 75(2):127–134
Faheem Du J, Bao J, Irshad S, Talib MA, Zheng H (2020) Efficient capture of phosphate and cadmium using biochar with multifunctional amino and carboxylic moieties: kinetics and mechanism. Water Air Soil Pollut 231(1):1–25
Ferreira SLC, de Jesus DS, Casella RJ, Costa ACS, de Carvalho MS, Santelli RE (1999) An on-line solid phase extraction system using polyurethane foam for the spectrophotometric determination of nickel in silicates and alloys. Anal Chim Acta 378(3):287–292
Feihu L, Bingtao T, Jinghai X, Shufen Z (2016) Hydrophilic modification of multi-walled carbon nanotube for building photonic crystals with enhanced color visibility and mechanical strength. Molecules 21(5):547
Fraczek-Szczypta A, Menaszek E, Blazewicz S, Menaszek E, Syeda TB, Misra A, Alavijeh M, Adu J (2012) Effect of MWCNT surface and chemical modification on in vitro cellular response. J Nanopart Res 14:1181–1194
Ghaedi M, Asadpour E (2006) Simultaneous preconcentration and determination of copper, nickel, cobalt, lead, and iron content using a surfactant-coated alumina. Bull Chem Soc Jpn 79(3):432–436
Gouda AA, Zordok WA (2018) Solid-phase extraction method for preconcentration of cadmium and lead in environmental samples using multiwalled carbon nanotubes Turk. J.Chem. 42:1018
Gupta B, Deep A, Malik P (2001) Extraction and recovery of cadmium using Cyanex 923. Hydrometallurgy 61(1):65–71
Gustavo Rocha De C, Ilton Luiz De A, Paulo Dos Santos R (2004) Synthesis, characterization and determination of the metal ions adsorption capacity of cellulose modified with p-aminobenzoic groups. Mater. Res 7(2):329–334
Jeffery GH, Bassett J, Mendham J, Denney RC (eds) (1996) Vogel’s Text book of Quantitative chemical analysis. Addision Weiley Longman, England
Kato K, Uchida E, Kang ET, Uyama Y, Ikada Y (2003) Polymer surface with graft chains. Prog Polym Sci 28(2):209–259
Krishna PG, Gladius JM, Rambabu U, Rao TP, Naidu GRK (2004) Preconcentrative separation of chromium (VI) species from chromium(III) by coprecipitation of its ethyl xanthate complex onto naphthalene. Talanta 63(3):541–546
Leon G, Guzman MA (2008) Facilitated transport of copper through bulk liquid membranes containing different carriers: compared kinetic study. Desalination 223(3):330–336
Li S (2015) Recyclable CNTs/Fe3O4 magnetic nanocomposites as adsorbents to remove bisphenol A from water and their regeneration. Chem Eng J 260:231–239
Li D, Li Z (2018) Adsorption of heavy metal tolerance strains to Pb2+ and Cd2+ in wastewater. Environ Sci Pollut Res 25:32156–32162
Li YH, Wang S, Wei J, Zhang X, Xu C, Luan Z (2002) Lead adsorption on carbon nanotubes. Chem Phys Lett 357(3-4):263–266
Li YH, Di Z, Ding J, Wu D, Luan Z, Zhu Y (2005) Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Wat Res 39(3):605–609
Liang HD, Han DM (2006) Multi-walled carbon nanotubes as sorbent for flow injection on-line microcolumn preconcentration coupled with flame atomic absorption spectrometry for determination of cadmium and copper. Anal Lett 39(11):2285–2295
Liang P, Ding Q, Song F (2005) Application of multiwalled carbon nanotubes as solid phaseextraction sorbent for preconcentration of trace copper in water samples. J Sep Sci 28(17):2339–2343
Lu C, Liu C (2006) Removal of nickel(II) from aqueous solution by carbon nanotubes. J Chem Technol Biotechnol 81(4):1932–1940
Mittal A (2016) Fabrication of MWCNTs/ThO2 nanocomposite and its adsorption behavior for the removal of Pb(II) metal from aqueous medium. Desalin Water Treat Sci Eng 57:21863–21869
Munoz J, Gallego M, Valcarcel M (2005) Speciation of organometallic compounds in environmetal samples by gas chromatography after flow preconcentration on fullerenes and nanotubes. Anal Chem 77(16):5389–5395
Obayomi KS, Bello JO, Yahya MD, ChukwunedumE AJB (2020) Statistical analyses on effective removal of cadmium and hexavalent chromium ions by multiwall carbon nanotubes (MWCNTs). Heliyon 6(6):04174
Pyrzynska K (2010) Carbon nanostructures for separation, preconcentration and speciation of metal ions. Trends in Anal Chem 29(7):718–727
Rao TP, Metilda P, Gladis JM (2005) Overview of analytical methodologies for sea water analysis: part I-metals. Crit Rev Anal Chem 35(4):247–288
Rashi G, Neeraj K, Elvis FK, Suprakas SR (2019) Efficient removal of Pb(II) and Cd(II) from industrial mine water by a hierarchical MoS2/SH-MWCNT nanocomposite. ACS Omega 4:13922–13955
Saeed MM, Ahmed R (2005) Solid phase sorption of microamount of Hg(II) onto 1-(2-thiazolylazo)-2-naphthol (TAN) loaded polyurethane foam. Radiochim Acta 93(3):177–185
Senthilnathan J, Mohan S, Palanivelu K (2005) Recovery of chromium from electroplating wastewater using DI 2-(Ethylhexyl) phosphoric acid Sep. Sci Tech 40:2125
Shim JK, Mukherjee M, Goswami SR (2019) Sustainable removal of pernicious arsenic and cadmium by a novel composite of MnO2 impregnated alginate beads: a cost-efective approach for wastewater treatment. J Environ Manag 234:8–20
Shemirani F, Abkenar SD (2004) Preconcentration and determination of trace nickel using 1-(2-pyridylazo)-2-naphtol (PAN) immobilized on surfactant-coated alumina. J Anal Chem 59(4):327–330
Starvin AM, Prasada Rao T (2004) Removal and recovery of mercury(II) from hazardous wastes using 1-(2-thiazolylazo)-2-naphthol functionalized activated carbon as solid phase extractant. J Hazard Mater B113(1):75–79
Subramanian KS (1988) Determination of chromium (III) and chromium (VI) by ammonium pyrrolidinecarbodithioate-methyl isobutyl ketone-furnace atomic absorption spectrometry. Anal Chem 60(1):11–15
Tarley CRT, Barbosa AF, Segatelli MG, Figueiredo EC, Luccas PO (2006) Highly improved sensitivity of TS-FF-AAS for Cd(II) determination at ng L− 1 levels using a simple flow injection minicolumn preconcentration system with multiwall carbon nanotubes. J Anal At Spectrom 21(3):1305–1313
Titus CE, Ambali SA, Abdulsalami SK, Eyitayo AA, Jimoh OT, Mercy TB, Shufeng B, Wiets DR (2021) Adsorption of Cr(VI), Ni(II), Fe(II) and Cd(II) ions by KIAgNPs decorated MWCNTs in a batch and fxed bed process. Sci. Rep 11:75
Tofighy MA, Mohammadi T (2011) Adsorption ofdivalent heavy metal ions from water using carbon nanotube sheets. J Hazard Mater 185(1):140–147
Turker AR, Baytak S (2004) Use of Escherichia coli immobilized on amberlite XAD-4 as a solid-phase extractor for metal preconcentration and determination by atomic absorption spectrometry. Anal Sci 20(5):329–334
Tuzen M, Parlar K, Soylak M (2005) Enrichment/separation of cadmium(II) and lead(II) in environmental samples by solid phase extraction. J Hazard Mater B 121(1):79–87
Vellaichamy S (2017) Adsorptive separation of copper, nickel, lead, zinc and cadmium from aqueous solution using MWCNTs impregnated with D2EHPA and prior to their determination by FAAS: kinetic and equilibrium studies. Sep Sci Tech 52:644
Vellaichamy S, Palanivelu K (2010) Speciation of chromium in aqueous samples by solid phase extraction using multiwall carbon nanotubes impregnated with D2EHPA. Indian J Chem 49A(1):882–890
Vellaichamy S, Palanivelu K (2011) Preconcentration and separation of copper, nickel and zinc in aqueous samples by flame atomic absorption spectrometry after column solid-phase extraction onto MWCNTs impregnated with D2EHPA-TOPO mixture. J Hazard Mater 185(3):1131–1139
Venkateswaran P, Palanivelu K (2004) Solvent extraction of hexavalent chromium with tetrabutyl ammonium bromide from aqueous solution. Sep Puri Technol 40(3):279–284
Zhang BL et al (2020) Mechanism study about the adsorption of Pb(II) and Cd(II) with iron-trimesic metal-organic frameworks. Chem Eng J 385:123507
Zhang M, Yudasaka M, Iijima S (2004) Diameter enlargement of single-wall carbo nanotubes by oxidation. J Phys Chem B 108(1):149–153
Zhao X, Song N, Jia Q, Zhou W (2009) Determination of Cu, Zn, Mn, and Pb by microcolumn packed with multiwalled carbon nanotubes on-line coupled with flame atomic absorption spectrometry. Microchim Acta 166(3):329–335
Zhongbing W, Wenbin X, Fanghui J, Zongwen Z, Kai Z, Hui L (2021) The selective adsorption performance and mechanism of multiwall magnetic carbon nanotubes for heavy metals in wastewater. Sci Rep 11:16878
Funding
This work was supported by Council of Scientific and Industrial Research (CSIR), New Delhi, India, for providing financial assistance under the award of Senior Research Fellowship scheme (No. 09/468/(0369)/2007EMR-I).
Author information
Authors and Affiliations
Contributions
Vellaichamy Sebastian: experimental design, data curation, writing original draft, read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest
Additional information
Responsible Editor: Ioannis A. Katsoyiannis
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 121 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sebastian, V. Adsorptive detoxification of Cd2+ and Pb2+ from wastewater using MWCNTs functionalized with -di-(2-ethyl hexyl phosphoric acid) and bis-(2,4,4-trimethyl pentyl) phosphonic acid. Environ Sci Pollut Res 30, 122979–122995 (2023). https://doi.org/10.1007/s11356-023-30808-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30808-x