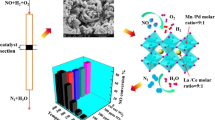
Abstract
The sulfur poisoning mechanism of low-temperature SCR de-NOx catalyst has always been one of the hot spots in academic circles. By studying the surface sulfur poisoning mechanism, low-temperature catalysts can be developed pertinently. In this paper, the mechanism of sulfur poisoning on the surface of LaMnO3 catalyst was studied by DFT method, and the adsorption process of sulfur oxides on the surface and its influence on SCR reaction process, as well as the morphology and decomposition process of ammonium sulfate on the surface were calculated. The results show that sulfur oxides will be adsorbed on the surface and occupy the adsorption site, which will adversely affect the subsequent SCR reaction. At the same time, ammonium sulfate will accumulate on the catalyst surface, which will lead to sulfur poisoning.














Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Bergner A, Dolg M, Küchle W, Stoll H, Preuß H (1993) Ab initio energy-adjusted pseudopotentials for elements of groups. Mol Phys 80:1431–1441
Chen X, Gui K, Gu S (2019) Catalyst denitration activity and sulfur resistance of modified siderite catalyst. J Fuel Chem Technol 47(3):370–377
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113(18):7756–7764
Forzatti P (2000) Environmental catalysis for stationary applications. Catal Today 62(1):51–65
Gavin AL, Watson GW (2017) Modelling oxygen defects in orthorhombic LaMnO3 and its low index surfaces. Phys Chem Chem Phys 19:24636–24646
Halgren TA, Lipscomb WN (1997) The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem Phys Lett 49:225–232
He G, He H (2016) DFT studies on the heterogeneous oxidation of SO2 by oxygen functional groups on graphene. Phys Chem Chem Phys 18(46):31691–31697
Larson L, Tao F (2001) Interactions and reactions of sulfur trioxide, water, and ammonia: an ab initio and density functional theory study. J Phys Chem A 105(17):4344–4350
Liu Y, Liu J, Lin YS, Chang M (2014) Effects of water vapor and trace gas impurities in flue gas on CO2/N2 separation using ZIF-8. Am Chem Soc. https://doi.org/10.1021/jp4113969
Lu W, Cui S, Guo H (2016) DRIFT and DFT study of cerium addition on SO2 of manganese-based catalysts for low temperature SCR. J Mol Catal a: Chem 4(1):102–108
Nakatsuji T, Akira M (1991) Removal technology for nitorogen oxides and sulfur oxides from exhaust gases. Catal Today 10(1):21–31
Ren D, Gui K, Gu S, Wei Y (2020) Study of the nitric oxide reduction of SCR-NH3 on nano-γFe2O3 catalyst surface with quantum chemistry. Appl Surf Sci 509:144659
Ren D, Gui K, Shaochen Gu (2021a) Comparison of sulfur poisoning resistance of Ce/Mn doped γ-Fe2O3 (001) surface in NH3-SCR reaction with DFT method. Appl Surf Sci 561:149847
Ren D, Gui K, Gu S (2021b) Mechanism of improving the SCR NO removal activity of Fe2O3 catalyst by doping Mn. J Alloys Compd 867:158787
Ren D, Gui K, Gu S (2021c) Quantum chemistry study of SCR-NH3 nitric oxide reduction on Ce-doped γFe2O3 catalyst surface. Mol Catal 502:111373
Royer S, Duprez D, Can (2014) Perovskites as substitutes of noble metals for heterogeneous catalysis: dream or reality. Chem Rev 114(20):10292–10368
Shi X, He H, Xie L (2015) The effect of Fe species distribution and acidity of Fe-ZSM-5 on the hydrothermal stability and SO2 and hydrocarbons durability in NH3-SCR reaction. Chin J Catal 36:649–656
Shi HY, Li XZ, Xia JW (2017) Sol-gel synthesis of LaBO3/attapulgite (B=Mn, Fe Co, Ni) nanocomposite for NH3-SCR of NO at low temperature. J Inorg Organomet Polym Mater 27(S1):166–172
Skalska K, Miller J, Ledakowicz S (2010) Trends in NO(x) abatement: a review. Sci Total Environ 408(19):3976–3989
Soyer S, Uzun A, Senkan S (2006) A quantum chemical study of nitric oxide reduction by ammonia (SCR reaction) on V2O5 catalyst surface. Catal Today 118(3–4):268–278
Wang Y, Chen L, Cao H, Chi Z, Chen C, Duan X, Xie Y, Qi F, Song W, Liu J, Wang S (2019) Role of oxygen vacancies and Mn sites in hierarchical Mn2O3/LaMnO3-δ perovskite composites for aqueous organic pollutants decontamination. Appl Catal B Environ 245:546–554
Wang Z, Liu J, Yang Y, Yu Y, Yan X, Zhang Z (2020) Insights into the catalytic behavior of LaMnO3 perovskite for Hg0 oxidation by HCl. J Hazard Mater 38:121156
Wei Y, Gui K, Liu X, Liang H, Gu S, Ren D (2019) Performance of Mn/Ce co-doped siderite catalysts in the selective catalytic reduction of NOx by NH3. J Fuel Chem Technol 47(12):1495–1503
Xu X, Gui K (2019) Effect of SCR denitrification performance with Mn and W doped siderite catalyst for diesel engine exhaust. Environ Eng 37(9):125–130
Yan X, Liu J, Yang Y et al (2021) A catalytic reaction scheme for NO reduction by CO over Mn-terminated LaMnO3 perovskite: a DFT study. Fuel Process Technol 216. https://doi.org/10.1016/j.fuproc.2021.106798
Yang JP, Zhang MG, Li HL (2018) Simultaneous NO reduction and Hg0 oxidation over La0.8Ce0.2MnO3 perovskite catalysts at low temperature. Ind Eng Chem Res 57(29):9374–9385
Yang Y, Liu J, Wang Z (2018) A skeletal reaction scheme for selective catalytic reduction of NO x with NH3 over CeO2/TiO2 catalyst. Fuel Process Technol 174:17–25
Yang Y, Liu J, Zhang B, Liu F (2017) Mechanistic studies of mercury adsorption and oxidation by oxygen over spinel-type MnFe2O4. Hazard Mater 321:154–161. https://doi.org/10.1016/j.jhazmat.2016.09.007
Yoosefian M, Zahedi M, Mola A (2015) A DFT comparative study of single and double SO2 adsorption on Pt-doped and Au-doped single-walled carbon nanotube. Appl Surf Sci 349:864–869
Zhang SB, Zhao YC (2018) Synergistic mercury removal over the CeMnO3 perovskite structure oxide as a selective catalytic reduction catalyst from coal combustion flue gas. Energy&fuels 32(11):11785–11795
Zhang RD, Luo N, Yang W (2013) Low-temperature selective catalytic reduction of NO with NH3 using perovskite-type oxides as the novel catalysts. J Mol Catal a: Chem 371:86–93
Zhu HY, Zhang PF, Dai S (2015) Recent advances of Lanthanum based perovskite oxides for catalysis. ACS Catal 5(11):6370–6385
Funding
This work was supported by the Natural Science Foundation of Shandong Province, China, (ZR2021QE295) and 2020 Science and Technology Project of Qingdao West Coast New Area (2020–99).
Author information
Authors and Affiliations
Contributions
Dongdong Ren: conceptualization, methodology, investigation, writing — original draft. Wencong Hao: data curation, formal analysis. Siyi Luo*: data curation, visualization. Wei Li: formal analysis. Pengyun Liu: formal analysis. Keting Gui: validation, formal analysis. Zongliang Zuo: writing — review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, D., Hao, W., Li, W. et al. Study of S poisoning mechanism on LaMnO3 perovskite catalyst surface based on DFT method. Environ Sci Pollut Res 30, 120315–120328 (2023). https://doi.org/10.1007/s11356-023-30498-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30498-5