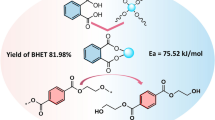
Abstract
In order to efficiently recycle waste polyethylene terephthalate (PET) bottles, this study aimed to enhance the hydrolysis process to convert PET bottle into valuable terephthalic acid (TPA) by developing effective and reusable Ni/γ-Al2O3 catalysts. A series of Ni/γ-Al2O3 catalyst was prepared by the impregnation method with different Ni loadings (5–15 wt%) and was characterized by various techniques including XRD, SEM-EDX, and N2 adsorption-desorption. The prepared catalysts were employed in the catalytic hydrolysis of PET under varied influencing factors, namely reaction temperature (220–280 °C), reaction time (20–60 min), and Ni loading. The response surface methodology (RSM) was used to optimize the operating condition to produce the maximum TPA yield, and the optimal values were determined as follows: reaction temperature = 267.07 °C, reaction time = 48.54 min, and Ni loading = 12.90 wt%, giving the highest TPA yield of 97.06%. The R2, F-value, and P-value of the analysis of variance (ANOVA) were 0.9982, 424.96, and <0.0001, respectively, indicating a good fit of the model. The results from XRD and FTIR measurement of the produced TPA indicated the high purity and comparable chemical structures to the TPA standard. In addition, the 12.9Ni/Al catalyst exhibited high catalytic activity in repeated cycles of hydrolysis process of PET and could be regenerated by calcination to restore its catalytic activity. This finding could be a promising alternative for an effective TPA recovery from waste plastic bottles.













Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bai B, Liu Y, Zhang H, Zhou F, Han X, Wang Q, Jin H (2020) Experimental investigation on gasification characteristics of polyethylene terephthalate (PET) microplastics in supercritical water. Fuel 262:116630. https://doi.org/10.1016/j.fuel.2019.116630
Borrelle SB et al (2020) Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 369:1515–1518. https://doi.org/10.1126/science.aba3656
Cao F, Wang LY, Zheng RR, Guo LY, Chen YM, Qian X (2022) Research and progress of chemical depolymerization of waste PET and high-value application of its depolymerization products. RSC Adv 12:31564–31576. https://doi.org/10.1039/d2ra06499e
Chawla S, Varghese BS, A C, Hussain CG, Keçili R, Hussain CM (2022): Environmental impacts of post-consumer plastic wastes: treatment technologies towards eco-sustainability and circular economy. Chemosphere 308, 135867 https://doi.org/10.1016/j.chemosphere.2022.135867
Chu J, Cai Y, Li C, Wang X, Liu Q, He M (2021) Dynamic flows of polyethylene terephthalate (PET) plastic in China. Waste Manag 124:273–282. https://doi.org/10.1016/j.wasman.2021.01.035
Čolnik M, Knez Ž, Škerget M (2021) Sub- and supercritical water for chemical recycling of polyethylene terephthalate waste. Chem Eng Sci 233:116389. https://doi.org/10.1016/j.ces.2020.116389
Dong X, Jin B, Kong Z, Sun Y (2020) Promotion effect of Re additive on the bifunctional Ni catalysts for methanation coupling with water gas shift of biogas: insights from activation energy. Chin J Chem Eng 28:1628–1636. https://doi.org/10.1016/j.cjche.2020.03.016
Echaroj S, Santikunaporn M, Phan AN (2023) Supercritical ethanol liquefaction of bamboo leaves using functionalized reduced graphene oxides for high quality bio-oil production. Renew Energy 204:848–857. https://doi.org/10.1016/j.renene.2022.12.110
Genta M, Iwaya T, Sasaki M, Goto M (2007) Supercritical methanol for polyethylene terephthalate depolymerization: observation using simulator. Waste Manag 27:1167–1177. https://doi.org/10.1016/j.wasman.2006.06.005
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3:e1700782. https://doi.org/10.1126/sciadv.1700782
Goto M (2009) Chemical recycling of plastics using sub- and supercritical fluids. J Supercrit Fluids 47:500–507. https://doi.org/10.1016/j.supflu.2008.10.011
Guo WZ, Lu H, Li XK, Cao GP (2016) Tungsten-promoted titania as solid acid for catalytic hydrolysis of waste bottle PET in supercritical CO2. RSC Adv 6:43171–43184. https://doi.org/10.1039/c6ra06298a
Harussani MM, Sapuan SM, Rashid U, Khalina A, Ilyas RA (2022) Pyrolysis of polypropylene plastic waste into carbonaceous char: priority of plastic waste management amidst COVID-19 pandemic. Sci Total Environ 803:149911. https://doi.org/10.1016/j.scitotenv.2021.149911
Hill WJ, Hunter WG (1966) A Review of response surface methodology: a literature survey. Technometrics 8:571–590. https://doi.org/10.1080/00401706.1966.10490404
Hossain MZ, Chowdhury MBI, Jhawar AK, Charpentier PA (2017) Supercritical water gasification of glucose using bimetallic aerogel Ru-Ni-Al2O3 catalyst for H2 production. Biomass Bioenergy 107:39–51. https://doi.org/10.1016/j.biombioe.2017.09.010
Kang MJ, Yu HJ, Jegal J, Kim HS, Cha HG (2020) Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst. Chem Eng J 398:125655. https://doi.org/10.1016/j.cej.2020.125655
Khalaf HI, Hasan OA (2012) Effect of quaternary ammonium salt as a phase transfer catalyst for the microwave depolymerization of polyethylene terephthalate waste bottles. Chem Eng J 192:45–48. https://doi.org/10.1016/j.cej.2012.03.081
Korhonen J, Honkasalo A, Seppälä J (2018) Circular economy: the concept and its limitations. Ecol Econ 143:37–46. https://doi.org/10.1016/j.ecolecon.2017.06.041
Kubiczek J, Derej W, Hadasik B, Matuszewska A (2023) Chemical recycling of plastic waste as a mean to implement the circular economy model in the European Union. J Clean Prod 406:136951. https://doi.org/10.1016/j.jclepro.2023.136951
Li X, Hong X (2018) Computational studies on Ni-catalyzed C−O bond activation of esters. J Organomet Chem 864:68–80. https://doi.org/10.1016/j.jorganchem.2018.01.019
Li XK, Lu H, Guo WZ, Cao GP, Liu HL, Shi YH (2015) Reaction kinetics and mechanism of catalyzed hydrolysis of waste PET using solid acid catalyst in supercritical CO2. AIChE J 61:200–214. https://doi.org/10.1002/aic.14632
Li YQ, Zhao ZG, Li T, Wang KG (2022) Influence of temperature, residence time, and solvent/feedstock mass ratio on overall product distribution and oil products quality in ethanol liquefaction of 230 polypropylene impact copolymer. Fuel 317. https://doi.org/10.1016/j.fuel.2022.123575
Lozano-Martinez P, Torres-Zapata T, Martin-Sanchez N (2021) Directing depolymerization of PET with subcritical and supercritical ethanol to different monomers through changes in operation conditions. ACS Sustain Chem Eng 9:9846–9853. https://doi.org/10.1021/acssuschemeng.1c02489
Nasution F, Husin H, Mahidin, Abnisa F, Tirta Yani F, Maulinda L, Ahmadi (2022): Conversion of pyrolysis vapors derived from non-biodegradable waste plastics (PET) into valuable fuels using nickel-impregnated HZSM5-70 catalysts. Energy Convers Manag 273, 116440 https://doi.org/10.1016/j.enconman.2022.116440
Pacheco-López A, Gómez-Reyes E, Graells M, Espuña A, Somoza-Tornos A (2023) Integrated synthesis, modeling, and assessment (iSMA) of waste-to-resource alternatives towards a circular economy: the case of the chemical recycling of plastic waste management. Comput Chem Eng 175:108255. https://doi.org/10.1016/j.compchemeng.2023.108255
Park Y, Shin DS, Woo SH, Choi NS, Shin KH, Oh SM, Lee KT, Hong SY (2012) Sodium terephthalate as an organic anode material for sodium ion batteries. Adv Mater 24:3562–3567. https://doi.org/10.1002/adma.201201205
Profeti LPR, Dias JAC, Assaf JM, Assaf EM (2009) Hydrogen production by steam reforming of ethanol over Ni-based catalysts promoted with noble metals. J Power Sources 190:525–533. https://doi.org/10.1016/j.jpowsour.2008.12.104
Purohit P, Seth K, Kumar A, Chakraborti AK (2017) C-O bond activation by nickel-palladium hetero-bimetallic nanoparticles for Suzuki-Miyaura reaction of bioactive heterocycle-tethered sterically hindered aryl carbonates. ACS Catal 7:2452–2457. https://doi.org/10.1021/acscatal.6b02912
Rodrigues Fernandes J, Pereira Amaro L, Curti Muniz E, Favaro SL, Radovanovic E (2020) PET depolimerization in supercritical ethanol conditions catalysed by nanoparticles of metal oxides. J Supercrit Fluids 158:104715. https://doi.org/10.1016/j.supflu.2019.104715
Seung-hoon K, Jae-sun J, Eun-hyeok Y, Kwan-Young L, Dong JM (2014) Hydrogen production by steam reforming of biomass-derived glycerol over Ni-based catalysts. Catal Today 228:145–151. https://doi.org/10.1016/j.cattod.2013.11.043
Singh V, Belova L, Singh B, Sharma YC (2018) Biodiesel production using a novel heterogeneous catalyst, magnesium zirconate (Mg2Zr5O12): process optimization through response surface methodology (RSM). Energy Convers Manag 174:198–207. https://doi.org/10.1016/j.enconman.2018.08.029
Su H, Kanchanatip E, Wang D, Zhang H, Antoni MI, Huang Z, Yan M (2020) Catalytic gasification of food waste in supercritical water over La promoted Ni/Al2O3 catalysts for enhancing H2 production. Int J Hydrog Energy 45:553–564. https://doi.org/10.1016/j.ijhydene.2019.10.219
Su H, Li T, Wang S, Zhu L, Hu Y (2023) Low-temperature upcycling of PET waste into high-purity H2 fuel in a one-pot hydrothermal system with in situ CO2 capture. J Hazard Mater 443:130120. https://doi.org/10.1016/j.jhazmat.2022.130120
Su HC, Li T, Zhu LJ, Wang SR (2021) Catalytic reforming of the aqueous phase derived from diluted hydrogen peroxide oxidation of waste polyethylene for hydrogen production. Chemsuschem 14:4270–4279. https://doi.org/10.1002/cssc.202100913
Sun Y, Liu S, Wang P, Jian X, Liao X, Chen W-Q (2022) China’s roadmap to plastic waste management and associated economic costs. J Environ Manag 309:114686. https://doi.org/10.1016/j.jenvman.2022.114686
Ugduler S, Van Geem KM, Denolf R, Roosen M, Mys N, Ragaert K, De Meester S (2020) Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green Chem 22:5376–5394. https://doi.org/10.1039/d0gc00894j
Vollmer I, Jenks MJF, Roelands MCP, White RJ, van Harmelen T, de Wild P, van der Laan GP, Meirer F, Keurentjes JTF, Weckhuysen BM (2020) Beyond mechanical recycling: giving new life to plastic waste. Angew Chem Int Ed 59:15402–15423. https://doi.org/10.1002/anie.201915651
Wang MH, He Y, Sen B (2019a) Research and management of plastic pollution in coastal environments of China. Environ Pollut 248:898–905. https://doi.org/10.1016/j.envpol.2019.02.098
Wang Y, Gu Y, Wu Y, Zhou G, Wang H, Han H, Chang T (2020) Performance simulation and policy optimization of waste polyethylene terephthalate bottle recycling system in China. Resour Conserv Recycl 162:105014. https://doi.org/10.1016/j.resconrec.2020.105014
Wang Y, Zhang Y, Song H, Wang Y, Deng T, Hou X (2019b) Zinc-catalyzed ester bond cleavage: chemical degradation of polyethylene terephthalate. J Clean Prod 208:1469–1475. https://doi.org/10.1016/j.jclepro.2018.10.117
Xayachak T, Haque N, Parthasarathy R, King S, Emami N, Lau D, Pramanik BK (2022) Pyrolysis for plastic waste management: an engineering perspective. J Environ Chem Eng 10:108865. https://doi.org/10.1016/j.jece.2022.108865
Xie S, Li L, Jin L, Wu Y, Liu H, Qin Q, Wei X, Liu J, Dong L, Li B (2020) Low temperature high activity of M (M = Ce, Fe, Co, Ni) doped M-Mn/TiO2 catalysts for NH3-SCR and in situ DRIFTS for investigating the reaction mechanism. Appl Surf Sci 515:146014. https://doi.org/10.1016/j.apsusc.2020.146014
Xin J, Zhang Q, Huang J, Huang R, Jaffery QZ, Yan D, Zhou Q, Xu J, Lu X (2021) Progress in the catalytic glycolysis of polyethylene terephthalate. J Environ Manag 296:113267. https://doi.org/10.1016/j.jenvman.2021.113267
Yabalak E (2018) Degradation of ticarcillin by subcritical water oxidation method: application of response surface methodology and artificial neural network modeling. J Environ Sci Health - Toxic/Hazard Subst Environ Eng 53:975–985. https://doi.org/10.1080/10934529.2018.1471023
Yan M, Liu J, Hantoko D, Kanchanatip E, Grisdanurak N, Cai Y, Gao Z (2019) Hydrogen-rich syngas production by catalytic cracking of tar in wastewater under supercritical condition. Int J Hydrog Energy 44:19908–19919. https://doi.org/10.1016/j.ijhydene.2019.05.234
Yan M, Yang Y, Shen T, Grisdanurak N, Pariatamby A, Khalid M, Hantoko D, Wibowo H (2023) Effect of operating parameters on monomer production from depolymerization of waste polyethylene terephthalate in supercritical ethanol. Process Saf Environ Prot 169:212–219. https://doi.org/10.1016/j.psep.2022.11.011
Yang W, Liu R, Li C, Song Y, Hu C (2021) Hydrolysis of waste polyethylene terephthalate catalyzed by easily recyclable terephthalic acid. Waste Manag 135:267–274. https://doi.org/10.1016/j.wasman.2021.09.009
Yang WS, Wang J, Jiao L, Song Y, Li C, Hu CQ (2022) Easily recoverable and reusable p-toluenesulfonic acid for faster hydrolysis of waste polyethylene terephthalate. Green Chem 24:1362–1372. https://doi.org/10.1039/D1GC04567A
Yang Y, Chen F, Shen T, Pariatamby A, Wen X, Yan M, Kanchanatip E (2023) Catalytic depolymerization of waste polyethylene terephthalate plastic in supercritical ethanol by ZnO/γ-Al2O3 catalyst. Process Saf Environ Prot 173:881–892. https://doi.org/10.1016/j.psep.2023.04.001
Yang Y, Lu YJ, Xiang HW, Xu YY, Li YW (2002) Study on methanolytic depolymerization of PET with supercritical methanol for chemical recycling. Polym Degrad Stab 75:185–191. https://doi.org/10.1016/s0141-3910(01)00217-8
Yao H, Liu L, Yan D, Zhou Q, Xin J, Lu X, Zhang S (2022) Colorless BHET obtained from PET by modified mesoporous catalyst ZnO/SBA-15. Chem Eng Sci 248:117109. https://doi.org/10.1016/j.ces.2021.117109
Yoshioka T, Sato T, Okuwaki A (1994) Hydrolysis of waste pet by sulfuric-acid at 150-degrees-c for a chemical recycling. J Appl Polym Sci 52:1353–1355. https://doi.org/10.1002/app.1994.070520919
Younis A, Estephane J, Gennequin C, Tidahy L, El Khoury B, Aouad S, Abi Aad E (2022) Influence of promoting Ni-based catalysts with ruthenium in the dry reforming of polypropylene plastics for syngas production. Int J Hydrog Energy 47:40204–40217. https://doi.org/10.1016/j.ijhydene.2022.07.156
Zhang LR, Gao J, Zou JZ, Yi FP (2013) Hydrolysis of poly(ethylene terephthalate) waste bottles in the presence of dual functional phase transfer catalysts. J Appl Polym Sci 130:2790–2795. https://doi.org/10.1002/app.39497
Zhao Z, Li Z, Zhang X, Li T, Li Y, Chen X, Wang K (2022) Catalytic hydrogenolysis of plastic to liquid hydrocarbons over a nickel-based catalyst. Environ Pollut 313:120154. https://doi.org/10.1016/j.envpol.2022.120154
Funding
This work was supported by the National Natural Science Foundation of China (52250410339 and 52150410422).
Author information
Authors and Affiliations
Contributions
The conceptualization and data curation were drawn by Mi Yan. Investigation, conducting experiment, data curation, and main manuscript were prepared by Yayong Yang. Figures, tables, and conducting experiment were done by Feng Chen. Methodology was designed by Agamuthu Pariatamby. Resources and research ideas were provided by Dwi Hantoko. Conceptualization, manuscript editing, and supervision were done by Ekkachai Kanchanatip. All authors read, reviewed, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, M., Yang, Y., Chen, F. et al. Development of reusable Ni/γ-Al2O3 catalyst for catalytic hydrolysis of waste PET bottles into terephthalic acid. Environ Sci Pollut Res 30, 102560–102573 (2023). https://doi.org/10.1007/s11356-023-29596-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29596-1