Abstract
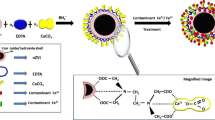
Chitosan-stabilized iron-copper nanomaterials (CS-nZVI/Cu) were successfully prepared and applied to the nitrate removal. Batch experiments were conducted to examine the effects of experimental parameters on nitrate removal, including Cu loading, CS-nZVI/Cu dosages, initial nitrate concentrations, and initial pHs. From the experimental date, it was concluded that CS-nZVI/Cu has a high nitrate removal efficiency, which can be more than 97%, respectively, at Cu loading = 5%, dosages of CS-nZVI/Cu = 3 g/L, initial nitrate concentrations of 30~120 mg/L, and initial pH values = 2~9. Additionally, the kinetic data for CS-nZVI/Cu were found to fit well with the first-order kinetic model with a rate constant of 0.15 (mg∙L)1-n/min, where n=1. The Langmuir model showed a good fit for NO3- removal, indicating that monolayer chemisorption occurred. The SEM and TEM analyses showed that the addition of chitosan resulted in improved dispersion of the CS-nZVI/Cu. The CS-nZVI/Cu nanomaterials have a more complete elliptical shape and are between 50 and 100 nm in size. The XRD analysis showed that the chitosan encapsulation reduced the oxidation of the iron component and the main product was Fe3O4. The FT-IR analysis showed that the immobilization of chitosan and the iron was accomplished by the ligand interaction. The nitrogen adsorption-desorption isotherm results showed that the CS-nZVI/Cu specific surface area and pore volume decreased significantly after the reaction. Adsorption, oxidation, and reduction are possible mechanisms for nitrate removal by CS-nZVI/Cu. The XPS analysis investigated the contribution of nZVI and Cu in the removal mechanism. Adding copper accelerates the reaction time and rate. In addition, nZVI played a vital role in reducing nitrate to N2. Based on these results, it looks like CS-nZVI/Cu could be a satisfactory material for nitrate removal.










Similar content being viewed by others
Data availability
All data and materials are true and valid and can be available by request.
References
Abascal E, Gómez-Coma L, Ortiz I, Ortiz A (2022) Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci Total Environ 810:152233
Aiello A, Morey JR, Livi KJT, DeLong HC, ElBidweihy H, Trulove PC, Durkin DP (2020) Lignocellulose-stabilized iron-palladium nanomagnetic biocomposites. J Magn Magn Mater 497:165964
Beigy MR, Rasekh B, Yazdian F, Aminzadeh B, Shekarriz M (2018) High nitrate removal by starch-stabilized Fe-0 nanoparticles in aqueous solution in a controlled system. Eng Life Sci 18:187–195
Bhatnagar A, Vilar VJP, Botelho CMS, Boaventura RAR (2011) A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Environ Technol 32:231–249
Cai X, Gao Y, Sun Q, Chen Z, Megharaj M, Naidu R (2014) Removal of co-contaminants Cu (II) and nitrate from aqueous solution using kaolin-Fe/Ni nanoparticles. Chem Eng J 244:19–26
Cecconet D, Devecseri M, Callegari A, Capodaglio AG (2018) Effects of process operating conditions on the autotrophic denitrification of nitrate-contaminated groundwater using bioelectrochemical systems. Sci Total Environ 613-614:663–671
Chen D, Wang D, Xiao Z, Wang H, Yang K (2018) Nitrate removal in a combined bioelectrochemical and sulfur autotrophic denitrification system under high nitrate concentration: effects of pH. Bioprocess Biosyst Eng 41:449–455
Chen Z-X, Jin X-Y, Chen Z, Megharaj M, Naidu R (2011) Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J Colloid Interface Sci 363:601–607
Choe S, Chang Y-Y, Hwang K-Y, Khim J (2000) Kinetics of reductive denitrification by nanoscale zero-valent iron. Chemosphere 41:1307–1311
Djouadi Belkada F, Kitous O, Drouiche N, Aoudj S, Bouchelaghem O, Abdi N, Grib H, Mameri N (2018) Electrodialysis for fluoride and nitrate removal from synthesized photovoltaic industry wastewater. Sep Purif Technol 204:108–115
Dong H, Zhao F, He Q, Xie Y, Zeng Y, Zhang L, Tang L, Zeng G (2017) Physicochemical transformation of carboxymethyl cellulose-coated zero-valent iron nanoparticles (nZVI) in simulated groundwater under anaerobic conditions. Sep Purif Technol 175:376–383
Duong HC, Cao HT, Hoang NB, Nghiem LD (2021) Reverse osmosis treatment of condensate from ammonium nitrate production: insights into membrane performance. J Environ Chem Eng 9:106457
Fang S, Zhang J, Niu Y, Ju S, Gu Y, Han K, Wan X, Li N, Zhou Y (2023) Removal of nitrate nitrogen from wastewater by green synthetic hydrophilic activated carbon supported sulfide modified nanoscale zerovalent iron: characterization, performance and mechanism. Chem Eng J 461:141990
Geng B, Jin Z, Li T, Qi X (2009) Kinetics of hexavalent chromium removal from water by chitosan-Fe0 nanoparticles. Chemosphere 75:825–830
Hamid S, Bae S, Lee W, Amin MT, Alazba AA (2015) Catalytic nitrate removal in continuous bimetallic Cu–Pd/nanoscale zerovalent iron system. Ind Eng Chem Res 54:6247–6257
Hamid S, Kumar MA, Han J-I, Kim H, Lee W (2017) Nitrate reduction on the surface of bimetallic catalysts supported by nano-crystalline beta-zeolite (NBeta). Green Chem 19:853–866
He K, Wang SC, Liu Y, Cao ZY, Yang LW, He F (2023) Enhanced removal of hexavalent chromium by lignosulfonate modified zero valent iron: reaction kinetic, performance and mechanism. Sci Total Environ 857
He Y, Lin H, Dong Y, Li B, Wang L, Chu S, Luo M, Liu J (2018) Zeolite supported Fe/Ni bimetallic nanoparticles for simultaneous removal of nitrate and phosphate: synergistic effect and mechanism. Chem Eng J 347:669–681
Hu Q, Chen N, Feng C, Hu W (2015) Nitrate adsorption from aqueous solution using granular chitosan-Fe3+ complex. Appl Surf Sci 347:1–9
Jiang D, Huang D, Lai C, Xu P, Zeng G, Wan J, Tang L, Dong H, Huang B, Hu T (2018) Difunctional chitosan-stabilized Fe/Cu bimetallic nanoparticles for removal of hexavalent chromium wastewater. Sci Total Environ 644:1181–1189
Jiang Z, Zhang S, Pan B, Wang W, Wang X, Lv L, Zhang W, Zhang Q (2012) A fabrication strategy for nanosized zero valent iron (nZVI)-polymeric anion exchanger composites with tunable structure for nitrate reduction. J Hazard Mater 233:1–6
Jin X, Li Q, Yang Q (2018) The reactivity of Fe/Ni colloid stabilized by carboxymethylcellulose (CMC-Fe/Ni) toward chloroform. Environ Sci Pollut Res 25:21049–21057
Kakavandi B, Kalantary RR, Farzadkia M, Mahvi AH, Esrafili A, Azari A, Yari AR, Javid AB (2014) Enhanced chromium (VI) removal using activated carbon modified by zero valent iron and silver bimetallic nanoparticles. J Environ Health Sci Eng 12:115
Kazemi M, Jahanshahi M, Peyravi M (2018) Hexavalent chromium removal by multilayer membrane assisted by photocatalytic couple nanoparticle from both permeate and retentate. J Hazard Mater 344:12–22
Li H, Ge Y, Zhang X (2017) High efficient removal of lead from aqueous solution by preparation of novel PPG-nZVI beads as sorbents. Colloids Surf A Physicochem Eng Asp 513:306–314
Li Q, Chen Z, Wang H, Yang H, Wen T, Wang S, Hu B, Wang X (2021) Removal of organic compounds by nanoscale zero-valent iron and its composites. Sci Total Environ 792:148546
Liou YH, Lo S-L, Lin C-J, Kuan WH, Weng SC (2005) Chemical reduction of an unbuffered nitrate solution using catalyzed and uncatalyzed nanoscale iron particles. J Hazard Mater 127:102–110
Liu A, Liu J, Zhang W-X (2015) Transformation and composition evolution of nanoscale zero valent iron (nZVI) synthesized by borohydride reduction in static water. Chemosphere 119:1068–1074
Liu T, Yang X, Wang Z-L, Yan X (2013) Enhanced chitosan beads-supported Fe0-nanoparticles for removal of heavy metals from electroplating wastewater in permeable reactive barriers. Water Res 47:6691–6700
Liu Y, Wang JL (2019) Reduction of nitrate by zero valent iron (ZVI)-based materials: a review. Sci Total Environ 671:388–403
Lubphoo Y, Chyan J-M, Grisdanurak N, Liao C-H (2016) Influence of Pd-Cu on nanoscale zero-valent iron supported for selective reduction of nitrate. J Taiwan Inst Chem Eng 59:285–294
Lyu Z, Liu W, Chi Z (2023a) Enhanced nitrate removal using in situ reactive zone with reduced graphene oxide supported nanoscale zero-valent iron. Environ Sci Pollut Res Intl
Lyu Z, Liu WT, Chi ZF (2023b) Enhanced nitrate removal using in situ reactive zone with reduced graphene oxide supported nanoscale zero-valent iron. Environ Sci Pollut Res
Ma Q, Teng W, Sun Y, Chen Y, Xue Y, Chen X, Zhang C, Zhang H, Fan J, Qiu Y, Fu R (2022) Multi-component removal of Pb(II), Cd(II), and As(V) over core-shell structured nanoscale zero-valent iron@mesoporous hydrated silica. Sci Total Environ 827:154329
Mohseni-Bandpi A, Kakavandi B, Kalantary RR, Azari A, Keramati A (2015) Development of a novel magnetite–chitosan composite for the removal of fluoride from drinking water: adsorption modeling and optimization. RSC Adv 5:73279–73289
Mossa Hosseini S, Ataie-Ashtiani B, Kholghi M (2011) Nitrate reduction by nano-Fe/Cu particles in packed column. Desalination 276:214–221
Mukherjee A, Zimmerman AR, Harris W (2011) Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 163:247–255
Nair V, Panigrahy A, Vinu R (2014) Development of novel chitosan–lignin composites for adsorption of dyes and metal ions from wastewater. Chem Eng J 254:491–502
Nakseedee P, Tanboonchuy V, Khemthong P, Grisdanurak N, Liao C-H (2017) Role of Cu on zero valent bimetallic Cu-Fe in arsenic removal with gas bubbling. Environ Prog Sustain Energy 36:1449–1457
Palansooriya KN, Kim S, Igalavithana AD, Hashimoto Y, Choi Y-E, Mukhopadhyay R, Sarkar B, Ok YS (2021) Fe(III) loaded chitosan-biochar composite fibers for the removal of phosphate from water. J Hazard Mater 415:125464
Pang H, Diao Z, Wang X, Ma Y, Yu S, Zhu H, Chen Z, Hu B, Chen J, Wang X (2019) Adsorptive and reductive removal of U(VI) by Dictyophora indusiate-derived biochar supported sulfide NZVI from wastewater. Chem Eng J 366:368–377
Peng L, Liu Y, Gao S-H, Chen X, Xin P, Dai X, Ni B-J (2015) Evaluation on the nanoscale zero valent iron based microbial denitrification for nitrate removal from groundwater. Sci Rep 5:12331
Peng YY, He SB, Gu XS, Yan P, Tang L (2021) Zero-valent iron coupled plant biomass for enhancing the denitrification performance of ecological floating bed. Bioresour Technol 341
Shubair T, Eljamal O, Khalil AME, Matsunaga N (2018) Multilayer system of nanoscale zero valent iron and nano-Fe/Cu particles for nitrate removal in porous media. Sep Purif Technol 193:242–254
Song N, Xu J, Cao Y, Xia F, Zhai J, Ai H, Shi D, Gu L, He Q (2020) Chemical removal and selectivity reduction of nitrate from water by (nano) zero-valent iron/activated carbon micro-electrolysis. Chemosphere, 248
Sparis D, Mystrioti C, Xenidis A, Papassiopi N (2013) Reduction of nitrate by copper-coated ZVI nanoparticles. Desalin Water Treat 51:2926–2933
Sri Shalini S, Joseph K (2013) Start-up of the SHARON and ANAMMOX process in landfill bioreactors using aerobic and anaerobic ammonium oxidising biomass. Bioresour Technol 149:474–485
Stefaniuk M, Oleszczuk P, Ok YS (2016) Review on nano zerovalent iron (nZVI): from synthesis to environmental applications. Chem Eng J 287:618–632
Suzuki T, Moribe M, Oyama Y, Niinae M (2012) Mechanism of nitrate reduction by zero-valent iron: equilibrium and kinetics studies. Chem Eng J 183:271–277
Tang T-T, Xing Q-J, Zhang S-H, Mu Y, Jiang X-H, Zhou Z-G, Xiao X, Zou J-P (2019) High selective reduction of nitrate into nitrogen by novel Fe-Cu/D407 composite with excellent stability and activity. Environ Pollut 252:888–896
Tien Hiep N, Karunakaran G, Konyukhov YV, Nguyen Van M, Karpenkov DY, Burmistrov IN (2021) Impact of iron on the Fe-Co-Ni ternary nanocomposites structural and magnetic features obtained via chemical precipitation followed by reduction process for various magnetically coupled devices applications. Nanomaterials, 11
Wang Z, Fu W, Hu L, Zhao M, Guo T, Hrynsphan D, Tatsiana S, Chen J (2021) Improvement of electron transfer efficiency during denitrification process by Fe-Pd/multi-walled carbon nanotubes: possessed redox characteristics and secreted endogenous electron mediator. Sci Total Environ 781:146686
Wei A, Ma J, Chen J, Zhang Y, Song J, Yu X (2018) Enhanced nitrate removal and high selectivity towards dinitrogen for groundwater remediation using biochar-supported nano zero-valent iron. Chem Eng J 353:595–605
Xie Y, Yi Y, Qin Y, Wang L, Liu G, Wu Y, Diao Z, Zhou T, Xu M (2016) Perchlorate degradation in aqueous solution using chitosan-stabilized zero-valent iron nanoparticles. Sep Purif Technol 171:164–173
Xu H, Li X, Gao M, Hu X, Zhang X, Li Y, Xu X, Hu J, Tang C, Hu X (2022) Chitosan and biochar synergize the efficient elimination of lead from wastewater by sulfidised nano-zero-valent iron. J Environ Chem Eng 10:107101
Yang J, Tian H, Guo J, He J (2023) 3D porous carbon-embedded nZVI@Fe2O3 nanoarchitectures enable prominent performance and recyclability in antibiotic removal. Chemosphere 331:138716
Yaragal RR, Mutnuri S (2022) Nitrates removal using ion exchange resin: batch, continuous column and pilot-scale studies. Intl J Environ Sci Technol
Ye JE, Wang Y, Xu Q, Wu HX, Tong JH, Shi JY (2021) Removal of hexavalent chromium from wastewater by Cu/Fe bimetallic nanoparticles. Scientific Reports, 11
Zhang D, Li Y, Tong S, Jiang X, Wang L, Sun X, Li J, Liu X, Shen J (2018) Biochar supported sulfide-modified nanoscale zero-valent iron for the reduction of nitrobenzene. RSC Adv 8:22161–22168
Zhang J, Niu Y, Zhou Y, Ju S, Gu Y (2022) Green preparation of nano-zero-valent iron-copper bimetals for nitrate removal: characterization, reduction reaction pathway, and mechanisms. Adv Powder Technol 33:103807
Zhang XR, Yan L, Liu JF, Zhang ZY, Tan CH (2019) Removal of different kinds of heavy metals by novel PPG-nZVI beads and their application in simulated stormwater infiltration facility. Appl Sci-Basel, 9
Zhao X, Liu W, Cai Z, Han B, Qian T, Zhao D (2016) An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res 100:245–266
Zhou J, Sun Q, Chen D, Wang H, Yang K (2017) Ochrobactrum anthropi used to control ammonium for nitrate removal by starch-stabilized nanoscale zero valent iron. Water Sci Technol 76:1827–1832
Funding
This work was financially supported by the National Key R&D Program of China (No. 2020YFC1806302), the Prospective Joint Research Project of Industry, University and Research in Jiangsu Province (BY2016005-11), and the National Science and Technology Support Plan (No. 2013BAE111B03).
Author information
Authors and Affiliations
Contributions
Xiaxia Yang: writing-original draft, data curation, investigation, conceptualization, methodology. Wenhong Yang: conceptualization, investigation, writing-review, resources. Yingjie Chen: resources, investigation, supervision. Zixi Li: investigation, resources, supervision. Gang Yang: funding acquisition, investigation, writing-review and editing, supervision, project administration.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent to publish
All the authors listed have approved the manuscript that is enclosed.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Yang, W., Chen, Y. et al. Chitosan-stabilized iron-copper nanoparticles for efficient removal of nitrate. Environ Sci Pollut Res 30, 97298–97309 (2023). https://doi.org/10.1007/s11356-023-29319-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29319-6