Abstract
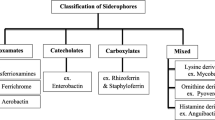
Siderophores have great application potential in metal pollutant remediation because of their effective cost and friendly impact on the environment. However, the practical use of siderophores in the remediation of specific metals is rather limited because of the weak nonspecific interactions between the siderophores and different metals. Thus, screening for a siderophore with optimal interaction with a specific metal would be necessary. In this study, the interaction between metal ions and moieties that donate the oxygen ligands for the coordination of four types of siderophore (hydroxamates, catecholates, phenolates, and carboxylates) was modeled and analyzed. As revealed by DFT-based analysis, the four types of siderophore generally exhibited selection preference for different metal ions in the order Ga3+ > Al3+ > Fe3+ > Cr3+ > Ni2+ > Cu2+ > Zn2+ > Co2+ > Mn2+ > Hg2+ > Pb2+ > Cd2+, which was determined mainly by the electronegativity of the siderophore functional groups, the electronegativity of the metals, and the ionic radius of the metals, as well as the interaction between the siderophores and the metals. Moreover, the effect of linear or nonlinear (cyclic) structure on the affinity of each siderophore for different metal ions was evaluated. In most situations, metal-bound cyclic siderophores were found to be more stable than their linear counterparts. Thus, proper siderophores for the remediation of metal pollution may be rapidly screened using this model.






Similar content being viewed by others
Data availability
Not applicable.
References
Ahmed E, Holmstrom SJM (2014) Siderophores in environmental research: roles and applications. Microb Biotechnol 7:196–208
Al Shaer D, Al Musaimi O, de la Torre BG, Albericio F (2020) Hydroxamate siderophores: natural occurrence, chemical synthesis, iron binding affinity and use as Trojan horses against pathogens. Eur J Med Chem 208:112791
Bailey DC, Bohac TJ, Shapiro JA, Giblin DE, Wencewicz TA, Gulick AM (2018) Crystal structure of the siderophore binding protein baub bound to an unusual 2:1 complex between acinetobactin and ferric iron. Biochemistry 57:6653–6661
Boukhalfa H, Crumbliss AL (2002) Chemical aspects of siderophore mediated iron transport. Biometals 15:325–339
Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP (2012) The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol 8:731–736
Clarke SE, Stuart J, Sanders-Loehr J (1987) Induction of siderophore activity in Anabaena spp. and its moderation of copper toxicity. Appl Environ Microbiol 53:917–922
Dev VV, Baburaj G, Antony S, Arun V, Krishnan KA (2020) Zwitterion-chitosan bed for the simultaneous immobilization of Zn(II), Cd(II), Pb(II) and Cu(II) from multi-metal aqueous systems. J Clean Prod 255:120309
Domagal-Goldman SD, Paul KW, Sparks DL, Kubicki JD (2009) Quantum chemical study of the Fe(III)-desferrioxamine B siderophore complex-electronic structure, vibrational frequencies, and equilibrium Fe-isotope fractionation. Geochim Cosmochim Acta 73:1–12
Ehlert S, Stahn M, Spicher S, Grimme S (2021) Robust and efficient implicit solvation model for fast semiempirical methods. J Chem Theory Comput 17:4250–4261
Hider RC, Kong XL (2010) Chemistry and biology of siderophores. Nat Prod Rep 27:637–657
Jackson VE, Felmy AR, Dixon DA (2015) Prediction of the pKa’s of aqueous metal ion +2 complexes. J Phys Chem A 119:2926–2939
Kirby ME, Sonnenberg JL, Simperler A, Weiss DJ (2020) Stability series for the complexation of six key siderophore functional groups with uranyl using density functional theory. J Phys Chem A 124:2460–2472
Kircheva N, Dudev T (2020) Gallium as an antibacterial agent: a DFT/SMD study of the Ga3+/Fe3+ competition for binding bacterial siderophores. Inorg Chem 59:6242–6254
Kurth C, Kage H, Nett M (2016) Siderophores as molecular tools in medical and environmental applications. Org Biomol Chem 14:8212
Levine DS, Head-Gordon M (2017) Energy decomposition analysis of single bonds within Kohn–Sham density functional theory. Proc Natl Acad of Sci 114:12649-12656
Lu T, Chen Q (2022) Independent gradient model based on Hirshfeld partition: a new method for visual study of interactions in chemical systems. J Comput Chem 43:539–555
Martell AE, Hancock RD (1996) Chelating Ligands.Metal complexes in aqueous solutions. Springer US, Boston, MA, pp. 63-95
McRose DL, Seyedsayamdost MR, Morel FMM (2018) Multiple siderophores: bug or feature? J Biol Inorg Chem 23:983–993
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–451
Moscatello N, Swayambhu G, Jones CH, Xu J, Dai N, Pfeifer BA (2018) Continuous removal of copper, magnesium, and nickel from industrial wastewater utilizing the natural product yersiniabactin immobilized within a packed-bed column. Chem Eng J 343:173–179
Oh J, Kang D, Hong S, Kim SH, Choi J-H, Seo J (2021) Formation of a tris(catecholato) iron(iii) complex with a nature-inspired cyclic peptoid ligand. Dalton Trans 50:3459–3463
Padial NM, Castells-Gil J, Almora-Barrios N, Romero-Angel M, da Silva I, Barawi M et al (2019) Hydroxamate titanium-organic frameworks and the effect of siderophore-type linkers over their photocatalytic activity. J Am Chem Soc 141:13124–13133
Pracht P, Bohle F, Grimme S (2020) Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys Chem Chem Phys 22:7169–7192
Pracht P, Caldeweyher E, Ehlert S, Grimme S (2019) A robust non-self-consistent tight-binding quantum chemistry method for large molecules. https://doi.org/10.26434/chemrxiv.8326202.v1
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149
Ribeiro M, Simoes M (2019) Advances in the antimicrobial and therapeutic potential of siderophores. Environ Chem Lett 17:1485–1494
Roskova Z, Skarohlid R, McGachy L (2022) Siderophores: an alternative bioremediation strategy? Sci Total Environ 819:153144
Ruetschlin S, Gunesch S, Boettcher T (2017) One enzyme, three metabolites: Shewanella algae controls siderophore production via the cellular substrate pool. Cell Chem Biol 24:598–604
Schmiederer T, Rausch S, Valdebenito M, Mantri Y, Mösker E, Baramov T et al (2011) The E coli siderophores enterobactin and salmochelin form six-coordinate silicon complexes at physiological pH. Angew Chem Int Ed 50:4230–4233
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Sinha V, Laan JJ, Pidko EA (2021) Accurate and rapid prediction of pKa of transition metal complexes: semiempirical quantum chemistry with a data-augmented approach. Phys Chem Chem Phys 23:2557–2567
Spicher S, Plett C, Pracht P, Hansen A, Grimme S (2022) Automated molecular cluster growing for explicit solvation by efficient force field and tight binding methods. J Chem Theory Comput 18:3174–3189
Tee GT, Gok XY, Yong WF (2022) Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: a review. Environ Res 212:113248
Teo SH, Ng CH, Islam A, Abdulkareem-Alsultan G, Joseph CG, Janaun J et al (2022) Sustainable toxic dyes removal with advanced materials for clean water production: a comprehensive review. J Clean Prod 332:130039
Wilson BR, Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y (2016) Siderophores in iron metabolism: from mechanism to therapy potential. Trends Mol Med 22:1077–1090
Winkelmann G (2002) Microbial siderophore-mediated transport. Biochem Soc Trans 30:691–696
Acknowledgements
We thank Professor Alan K Chang (Wenzhou University) for revising the language of the manuscript.
Funding
This work was supported by Zhejiang Provincial Natural Science Foundation of China [grant number LY23D060001], the National Natural Science Foundation of China [grant number 31670402], and the National Key R&D Program of China [grant number 2018YFD0901504].
Author information
Authors and Affiliations
Contributions
Xu Yi-Cheng: methodology, software, investigation, and writing—original draft. Li Nan: resources and visualization. Yan Xiufeng: conceptualization and supervision. Zou Hui-Xi: conceptualization, writing—review and editing, and funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, YC., Li, N., Yan, X. et al. DFT-based analysis of siderophore-metal ion interaction for efficient heavy metal remediation. Environ Sci Pollut Res 30, 91780–91793 (2023). https://doi.org/10.1007/s11356-023-28854-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28854-6