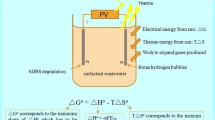
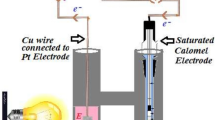
Abstract
Actual plan of research work was proposed for systematic investigating in the field of photogalvanic (PG) cells for solar energy transformation. It was necessary and proposed to carry out experimental work under the solar parameters for PG cells. The object of the research work is to enhance the solar energy conversion into electricity and store it through PG cells. Various parameters were studied in a PG cell having D-Xylose + MB + Brij-35 + NaLS system (mixed surfactants). In this study, the observed optimum results in terms of the open circuit voltage, photopotential, maximum photocurrent, and short circuit current are 921.00 mV, 698.00 mV, 311 uA, and 245.0 uA, respectively. The observed equilibrium photocurrent, current at power point, fill factor, and conversion efficiency were 243.0 uA and 142.0 uA, 0.4521, and 0.6769%, respectively. For individual surfactants, the observed results in terms of the open circuit voltage, photopotential, maximum photocurrent, and short circuit current are 870.00 mV, 635.00 mV, 175 uA, and 90.0 uA, respectively. For individual surfactant system, the observed equilibrium photocurrent, current at power point, fill factor, and conversion efficiency were 84.0 uA and 55.0 uA, 0.3630, and 0.3100%, respectively. The impact of solar energy was studied by varying the various parameters in PG cells. On the basis of above obtained values, the mixed surfactants (NaLS + Brij-35) have experimentally proved the efficient system as the desired object of research with special reference to enhance electrical out and storage of solar energy.





Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- i eq :
-
Photocurrent at equilibrium
- i sc :
-
Short circuit current
- i pp :
-
Photocurrent at power point
- mV :
-
Millivolt
- ml :
-
Milliliter
- M :
-
Molarity
- t 1/2 :
-
Storage capacity of cell
- pp :
-
Power point
- V pp :
-
Photopotential at power point
- V oc :
-
Open circuit voltage
- µA :
-
Microampere
- η :
-
Fill factor
- uW :
-
Microwatt
- PGS :
-
Photogalvanic system
- PG :
-
Photogalvanic
- i max :
-
Maximum photocurrent
- MB :
-
Methylene blue
- NaLS :
-
Sodium lauryl sulphate
References
Ageev AA, Volkov BA, Kibalov MS, Kukleva KK (2012) Correlation between wetting and deterging abilities in mixed surfactant solutions. Fibre Chem 44:17
Albery WJ, Archer MD (1977) Optimum efficiency of photogalvanic cells for solar energy conversion. Nature 270:399
Bayer LS, Erogle I, Turker L (2001) Photogalvaniceffectin aqueous methylene blue–nickel mesh system: conversion of light into electricity. Int J Energy Res 25:207
Bhimwal MK, Gangotri KM, Bhimwal MK (2013) A comparison of conversion efficiencies of various sugars as reducing agents for the photosensitizer eosin in the photogalvanic cell. Int J Energy Res 37:250
Chen WH, Tsai ZL, Chang MH, Hsu TH, Kuo PC (2022) Flow field simulation and pressure drop modeling by a porous medium in PEM fuel cells. Int J Energy Res 46:163
Das D, Kamble AD, Kalita P (2022) Performance investigation of transparent photovoltaic-thermal collector with horizontal oscillating and rectangular spiral flow patterns. Int J Energy Res 46(1):239
Gangotri P, Gangotri KM (2010) Studies of the micellar effect on photogalvanics: solar energy conversion and storage–EDTA–safranine O– DSS system. Int J Energy Res 34:1155
Gangotri KM, Mohan L (2013) Study of photogalvanic effect in photogalvanic cell containing mixed surfactant (NaLS+CTAB), methylene blue as a photosensitizer and xylose as reductant. Res J Chem Sci 3:20
Hall DE, Clark WDK, Eckert JA, Lichtin NN, Wildes PD (1977) A photogalvanic cell with semiconductor anode. Am Ceram Soc Bull 56:408
Jayshree R, Mohan L (2018) Study of photogalvanic effect in photogalvanic cell containing single surfactant as DSS, Tatrazine as a photosensitizer and EDTA as reductant for solar energy conversion and storage. Res J Chem Environ 22(6):53-57
Koli P, Dayma Y, Pareek RK, Kumar R, Jonwal M (2022) Modified and simplified photogalvanic cells: solar energy harvesting using bromo cresol green dye with different electrodes and cell dimensions. J Electroanal Chem 904:115942
Lal C (2007) Use of mixed dyes in a photogalvanic cell for solar energy conversion and storage: EDTA thionine- Azur B system. J Power Sources 164:926
Lal M, Gangotri KM (2013a) Study of photogalvanic effect in photogalvanic cell containing mixed surfactant (NaLS+Tween-80), methylene blue as a photosensitizer and xylose as reductant. Res J Resent Sci 2:76
Lal M, Gangotri KM (2013b) A comparative study on the performance of photogalvanic cell with mixed surfactant for solar energy conversion and storage. Res J Resent Sci 2:19
Lee NM, Lee BH (2012) Mixed micellization of TTAB with other surfactants (DTAB, CTAB, Tween-20, Tween-40, and Tween-80). J Korean Chem Soc 56:556
Mao S, Fan D, Shen W (2013) Influence of the mixed micelles on the electron transfer reaction [Co(NH3)5Cl]2 ++ [Fe(CN)6]4- . Colloids and Surfaces A: Physicochemical and Engineering Aspects 420:103–108
Memming R (1980) Solar energy conversion by photoelectrochemical processes. Electrochimica Acta 25:77
Murthy ASN, Dak AC, Reddy KS (1980) Photogalvanic effect in riboflavin ethylenediaminetetraacetic acid system. Int J Energy Res 4:339
Rabinowitch E (1940) The photogalvanic effect I. The photochemical properties of the thionine-iron system. J Chem Phys 8:551
Rathore J, Rakesh Kumar A, Sharma P, Lal M (2022) Study of electrical output in photogalvanic cell for solar energy conversion and storage: lauryl glucoside-tartrazine-D-fructose system. Indian J Sci Technol 15(23):1159
Rideal EK, Williams EG (1925) The action of light on the ferrous ferric iodine iodide equilibrium. J Chem Soc Trans 127:258
Suda Y, Shimoura Y, Sakata T, Tsubomura H (1978) Photogalvanic effect in the thionine iron system at semiconductor electrodes. J Phys Chem 82:268
Thareja P, Golematis A, Street BC, Wagner JN, Vethamuthu MS, Hermanson KD (2013) Influence of surfactants on the rheology and stability of crystallizing fatty acid pastes. J Am Oil Chem Soc 90:273
Wildes PD, Hobart DR, Lichtin NN, Hall DE, Eckert JA (1977) Sensitization of an iron–thiazine photogalvanic cell to the blue: an improved match to the insulation’s spectrum. Sol Energy 19:567
Xiang M, Lu Z, You Z et al (2022) Interaction quantitative modeling of mixed surfactants for synergistic solubilization by resonance light scattering. Environ Sci Pollut Res 29:11874
Zhang D, Shen B, Zhang M et al (2023) Surfactant recovery and efficient separation of PAHs from surfactant solutions by low-cost waste activated sludge and two-stage design optimization. Environ Sci Pollut Res 30:50484
Acknowledgements
The authors are thankful to Jai Narain Vyas University, Jodhpur Rajasthan, India, for laboratory facilities.
Author information
Authors and Affiliations
Contributions
All authors (Mohan Lal and KM Gangotri) contributed to the study conception, design, material preparation, data collection, and analysis.
Corresponding author
Ethics declarations
Ethical approval
We all authors read and approved the final manuscript.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The photogalvanic is emerging field of research.
• Developed photogalvanic cell with special attention to better performance.
• Reduces the cost of the photogalvanic cell for its commercial viability.
• Manuscript contains substantial electrical output, conversion efficiency and storage.
• Global scientific community is compelled to search out the renewable source of energy to feed the whole world.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lal, M., Gangotri, K.M. Innovative study in renewable energy source through mixed surfactant system for eco-friendly environment. Environ Sci Pollut Res 30, 98805–98813 (2023). https://doi.org/10.1007/s11356-023-28246-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28246-w