Abstract
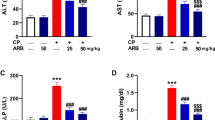
Cisplatin (CIS) is an effective chemotherapy against different solid cancers. However, the adverse effects, including hepatotoxicity, limit its clinical use. 7-hydroxycoumarin (7-HC) possesses antioxidant and hepatoprotective activities, but its protective effect against CIS hepatotoxicity has not been investigated. This study evaluated the effect of 7-HC on liver injury, oxidative stress (OS), and inflammation provoked by CIS. Rats received 7-HC (25, 50, and 100 mg/kg) orally for 2 weeks followed by intraperitoneal injection of CIS (7 mg/kg) at day 15. CIS increased serum transaminases, alkaline phosphatase (ALP), and bilirubin and provoked tissue injury accompanied by elevated reactive oxygen species (ROS), malondialdehyde (MDA), and nitric oxide (NO). Liver nuclear factor (NF)-κB p65, inducible NO synthase (iNOS), pro-inflammatory cytokines, Bax, and caspase-3 were upregulated, and antioxidant defenses and Bcl-2 were decreased in CIS-treated rats, while 7-HC prevented liver injury and ameliorated OS, inflammatory and apoptosis markers. In addition, 7-HC enhanced nuclear factor erythroid 2–related factor 2 (Nrf2), and heme oxygenase (HO)-1 in CIS-administered rats and in silico studies revealed its binding affinity toward HO-1. In conclusion, 7-HC protected against CIS hepatotoxicity by mitigating OS and inflammatory response and modulating Nrf2/HO-1 pathway.







Similar content being viewed by others
Data availability
The manuscript and supplementary material contain all data supporting the reported results.
References
Abd Rashid N, Abd Halim SAS, Teoh SL, Budin SB, Hussan F, Adib Ridzuan NR, Abdul Jalil NA (2021) The role of natural antioxidants in cisplatin-induced hepatotoxicity. Biomed Pharmacother 144:112328
Abraham NG, Lutton JD, Levere RD (1985) Heme metabolism and erythropoiesis in abnormal iron states: role of delta-aminolevulinic acid synthase and heme oxygenase. Exp Hematol 13:838–843
Aithal GP (2015) Pharmacogenetic testing in idiosyncratic drug-induced liver injury: current role in clinical practice. Liver Int 35:1801–1808
Aladaileh SH, Al-Swailmi FK, Abukhalil MH, Ahmeda AF, Mahmoud AM (2021) Punicalagin prevents cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammatory response, and apoptosis in rats. Life Sci 286:120071
Antar SA, Abdo W, Taha RS, Farage AE, El-Moselhy LE, Amer ME, Abdel Monsef AS, Abdel Hamid AM, Kamel EM, Ahmeda AF, Mahmoud AM (2022) Telmisartan attenuates diabetic nephropathy by mitigating oxidative stress and inflammation, and upregulating Nrf2/HO-1 signaling in diabetic rats. Life Sci 291:120260
Auten RL, Davis JM (2009) Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res 66:121–127
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 6th edition. Churchill Livingstone, Elsevier, China
Bernal W, Wendon J (2013) Acute liver failure. N Engl J Med 369:2525–2534
Brozovic A, Ambriović-Ristov A, Osmak M (2010) The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Crit Rev Toxicol 40:347–359
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–38
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Florea AM, Büsselberg D (2011) Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (basel) 3:1351–1371
Ghosh S (2019) Cisplatin: The first metal based anticancer drug. Bioorg Chem 88:102925
Gibson-Corley KN, Olivier AK, Meyerholz DK (2013) Principles for valid histopathologic scoring in research. Vet Pathol 50:1007–1015
Gozzelino R, Jeney V, Soares MP (2010) Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50:323–354
Green DR (1998) Apoptotic pathways: the roads to ruin. Cell 94:695–698
Greish KF, Salerno L, Al Zahrani R, Amata E, Modica MN, Romeo G, Marrazzo A, Prezzavento O, Sorrenti V, Rescifina AJM (2018) Novel structural insight into inhibitors of heme oxygenase-1 (HO-1) by new imidazole-based compounds: biochemical and in vitro anticancer activity evaluation. Molecules 23:1209
Grigorian A, O’Brien CB (2014) Hepatotoxicity secondary to chemotherapy. J Clin Transl Hepatol 2:95–102
Grisham MB, Johnson GG, Lancaster JR Jr (1996) Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol 268:237–246
Hassan HM, Al-Wahaibi LH, Elmorsy MA, Mahran YF (2020) Suppression of cisplatin-induced hepatic injury in rats through alarmin high-mobility group box-1 pathway by Ganoderma lucidum: theoretical and experimental study. Drug Des Devel Ther 14:2335–2353
Hozayen WG, Mahmoud AM, Desouky EM, El-Nahass ES, Soliman HA, Farghali AA (2019) Cardiac and pulmonary toxicity of mesoporous silica nanoparticles is associated with excessive ROS production and redox imbalance in Wistar rats. Biomed Pharmacother 109:2527–2538
Jansen T, Daiber A (2012) Direct antioxidant properties of bilirubin and biliverdin. Is there a role for biliverdin reductase? Front Pharmacol 3:30
Kim KM, Pae HO, Zheng M, Park R, Kim YM, Chung HT (2007) Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res 101:919–927
Kim M-J, Sim M-O, Lee H-I, Ham JR, Seo K-I, Lee M-K (2014) Dietary umbelliferone attenuates alcohol-induced fatty liver via regulation of PPARα and SREBP-1c in rats. Alcohol 48:707–715
Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M (2016) Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7:11624
Li MH, Jang JH, Na HK, Cha YN, Surh YJ (2007) Carbon monoxide produced by heme oxygenase-1 in response to nitrosative stress induces expression of glutamate-cysteine ligase in PC12 cells via activation of phosphatidylinositol 3-kinase and Nrf2 signaling. J Biol Chem 282:28577–28586
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Lv H, Xiao Q, Zhou J, Feng H, Liu G, Ci X (2018) Licochalcone A upregulates Nrf2 antioxidant pathway and thereby alleviates acetaminophen-induced hepatotoxicity. Front Pharmacol 9:147
Mahmoud AM, Germoush MO, Alotaibi MF, Hussein OE (2017a) Possible involvement of Nrf2 and PPARgamma up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed Pharmacother 86:297–306
Mahmoud AM, Hussein OE, Hozayen WG, Abd El-Twab SM (2017b) Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARgamma and Nrf2: protective effect of 18beta-glycyrrhetinic acid. Chem Biol Interact 270:59–72
Mahmoud AM, Mohammed HM, Khadrawy SM, Galaly SR (2017c) Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARgamma and TGF-beta1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem Biol Interact 277:146–158
Mahmoud AM, Wilkinson FL, Jones AM, Wilkinson JA, Romero M, Duarte J, Alexander MY (2017d) A novel role for small molecule glycomimetics in the protection against lipid-induced endothelial dysfunction: involvement of Akt/eNOS and Nrf2/ARE signaling. Biochim Biophys Acta Gen Subj 1861:3311–3322
Mahmoud AM, Hozayen WG, Hasan IH, Shaban E, Bin-Jumah M (2019) Umbelliferone ameliorates CCl4-induced liver fibrosis in rats by upregulating PPARγ and attenuating oxidative stress, inflammation, and TGF-β1/Smad3 signaling. Inflammation 42:1103–1116
Man Q, Deng Y, Li P, Ma J, Yang Z, Yang X, Zhou Y, Yan X (2020) Licorice ameliorates cisplatin-induced hepatotoxicity through antiapoptosis, antioxidative stress, anti-inflammation, and acceleration of metabolism. Front Pharmacol 11:563750
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Matata BM, Galiñanes M (2002) Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J Biol Chem 277:2330–2335
Mazimba O (2017) Umbelliferone: sources, chemistry and bioactivities review. Bull Fac Pharm Cairo Univ 55:223–232
McKim SE, Gäbele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, Mason RP, Doll MA, Hein DW, Arteel GE (2003) Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology 125:1834–1844
Mirzaei S, Mohammadi AT, Gholami MH, Hashemi F, Zarrabi A, Zabolian A, Hushmandi K, Makvandi P, Samec M, Liskova A, Kubatka P, Nabavi N, Aref AR, Ashrafizadeh M, Khan H, Najafi M (2021) Nrf2 signaling pathway in cisplatin chemotherapy: potential involvement in organ protection and chemoresistance. Pharmacol Res 167:105575
Moore TJ, Cohen MR, Furberg CD (2007) Serious adverse drug events reported to the Food and Drug Administration, 1998–2005. Arch Intern Med 167:1752–1759
Mudd TW, Guddati AK (2021) Management of hepatotoxicity of chemotherapy and targeted agents. Am J Cancer Res 11:3461–3474
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pan H, Wang H, Wang X, Zhu L, Mao L (2012) The absence of Nrf2 enhances NF-kappaB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediators Inflamm 2012:217580
Salerno L, Amata E, Romeo G, Marrazzo A, Prezzavento O, Floresta G, Sorrenti V, Barbagallo I, Rescifina A, Pittalà VJEjomc (2018) Potholing of the hydrophobic heme oxygenase-1 western region for the search of potent and selective imidazole-based inhibitors. Euro J Med Chem 148:54–62
Satta S, Mahmoud AM, Wilkinson FL, Yvonne Alexander M, White SJ (2017) The role of Nrf2 in cardiovascular function and disease. Oxid Med Cell Longev 2017:9237263
Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH (2009) Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci U S A 106:5171–5176
Shi Y, Chen J, Weng C, Chen R, Zheng Y, Chen Q, Tang H (2003) Identification of the protein-protein contact site and interaction mode of human VDAC1 with Bcl-2 family proteins. Biochem Biophys Res Commun 305:989–996
Sim MO, Lee HI, Ham JR, Seo KI, Kim MJ, Lee MK (2015) Anti-inflammatory and antioxidant effects of umbelliferone in chronic alcohol-fed rats. Nutr Res Pract 9:364–369
Siow RC, Sato H (1999) Mann GE (1999): Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res 41(2):385–394
Thomsen ND, Koerber JT, Wells JA (2013) Structural snapshots reveal distinct mechanisms of procaspase-3 and-7 activation. Proc Natl Acad Sci 110:8477–8482
Wardyn JD, Ponsford AH, Sanderson CM (2015) Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans 43:621–626
Yin J, Wang H, Lu G (2018) Umbelliferone alleviates hepatic injury in diabetic db/db mice via inhibiting inflammatory response and activating Nrf2-mediated antioxidant. Biosci Rep 38:BSR20180444
Zhang Y, Tao X, Yin L, Xu L, Xu Y, Qi Y, Han X, Song S, Zhao Y, Lin Y, Liu K, Peng J (2017) Protective effects of dioscin against cisplatin-induced nephrotoxicity via the microRNA-34a/sirtuin 1 signalling pathway. Br J Pharmacol 174:2512–2527
Acknowledgements
Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2023R381), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2023R381), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.M.M., A.A.K., and A.S.S.; data curation: D.H.S., A.M.M., A.S.S., and E.M.K.; formal analysis: A.M.M.; investigation: D.H.S., A.M.M., A.S.S., E.M.K., and E.H.M.H.; methodology: D.H.S., A.M.M., A.S.S., E.M.K., and E.H.M.H.; project administration: R.S.A., A.M.M., and A.A.K.; resources: A.M.M., R.S.A., M.A.E., and E.M.K.; supervision: A.M.M.; validation: A.M.M.; visualization: A.M.M.; writing — original draft: D.H.S., A.S.S., A.M.M., and E.M.K.; writing — review and editing: A.M.M.
Corresponding author
Ethics declarations
Ethical approval
All animal experiments and procedures were approved by the Institutional Research Ethics Committee of Beni-Suef University (Ethical approval no.: 020–90).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sami, D.H., Soliman, A.S., Khowailed, A.A. et al. The protective effect of 7-hydroxycoumarin against cisplatin-induced liver injury is mediated via attenuation of oxidative stress and inflammation and upregulation of Nrf2/HO-1 pathway. Environ Sci Pollut Res 30, 80181–80191 (2023). https://doi.org/10.1007/s11356-023-27879-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27879-1