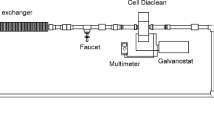
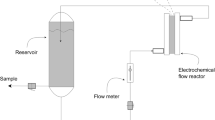
Abstract
Our previous study indicated excellent dechlorination efficiency and phenol conversion rate in the electrocatalytic reduction of 2,4-dichlorophenol (2,4-DCP) with a Pd-MWCNTs/Ni-foam electrode; it is deserved to investigate whether this electrode can efficiently degrade phenol in electro-Fenton oxidation (EFO) process and realize the effective mineralization of 2,4-DCP in aqueous solution. In this work, the sequential electrocatalytic reduction and oxidation of 2,4-DCP were studied after examining phenol degradation in the EFO process. The results showed that the removal efficiency of 0.31 mM phenol could reach 96.76% after 90-min degradation with the rate constant of 0.0367 min−1, and hydroxy radicals (·OH) were the main active species in the EFO process. In the sequential electrocatalytic reduction and oxidation processes, the removal efficiencies of 2,4-DCP, phenol, and total organic carbon (TOC) reached 99.72%, 97.07%, and 61.45%, respectively. The possible degradation mechanism of 2,4-DCP was proposed through monitoring the reaction products, and the stability and reusability of the electrode were also examined. This study suggested that 2,4-DCP in wastewater can be effectively mineralized to realize its efficient degradation through the sequential electrocatalytic reduction and oxidation.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Ahn YY, Bae H, Kim HI, Kim SH, Kim JH, Lee SG, Lee J (2019) Surface-loaded metal nanoparticles for peroxymonosulfate activation: efficiency and mechanism reconnaissance. Appl Catal B 241:561–569
Ahn YY, Yun ET, Seo JW, Lee C, Kim SH, Kim JH, Lee J (2016) Activation of peroxymonosulfate by surface-loaded noble metal nanoparticles for oxidative degradation of organic compounds. Environ Sci Technol 50:10187–10197
Babuponnusami A, Muthukumar K (2012) Advanced oxidation of phenol: a comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem Eng J 183:1–9
Burbano AA, Dionysiou DD, Suidan MT, Richardson TL (2005) Oxidation kinetics and effect of pH on the degradation of MTBE with Fenton reagent. Water Res 39:107–118
Cai J, Zhou M, Pan Y, Du X, Lu X (2019) Extremely efficient electrochemical degradation of organic pollutants with co-generation of hydroxyl and sulfate radicals on Blue-TiO2 nanotubes anode. Appl Catal B 257:117902
Chai Y, Qin P, Zhang J, Li T, Dai Z, Wu Z (2020) Simultaneous removal of Fe(II) and Mn(II) from acid mine wastewater by electro-Fenton process. Process Saf Environ 143:76–90
Chen Z, Liu Y, Wei W, Ni BJ (2019) Recent advances in electrocatalysts for halogenated organic pollutant degradation. Environ Sci -Nano 6:2332–2366
Eslami A, Hashemi M, Ghanbari F (2018) Degradation of 4-chlorophenol using catalyzed peroxymonosulfate with nano-MnO2/UV irradiation: toxicity assessment and evaluation for industrial wastewater treatment. J Clean Prod 195:1389–1397
Garba ZN, Zhou W, Lawan I, Xiao W, Zhang M, Wang L, Chen L, Yuan Z (2019) An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: a review. J Environ Manage 241:59–75
Gümüş D, Akbal F (2016) Comparison of Fenton and electro-Fenton processes for oxidation of phenol. Process Saf Environ 103:252–258
Hama Aziz KH, Miessner H, Mueller S, Mahyar A, Kalass D, Moeller D, Khorshid I, Rashid MAM (2018) Comparative study on 2, 4-dichlorophenoxyacetic acid and 2, 4-dichlorophenol removal from aqueous solutions via ozonation, photocatalysis and non-thermal plasma using a planar falling film reactor. J Hazard Mater 343:107–115
Han B, Liu W, Li J, Wang J, Zhao D, Xu R, Lin Z (2017) Catalytic hydrodechlorination of triclosan using a new class of anion-exchange-resin supported palladium catalysts. Water Res 120:199–210
Jadbabaei N, Ye T, Shuai D, Zhang H (2017) Development of palladium-resin composites for catalytic hydrodechlorination of 4-chlorophenol. Appl Catal B 205:576–586
Ji Y, Yang Y, Wang L, Lu J, Ferronato C, Chovelon JM (2018) Sulfate radical-induced incorporation of NO2 group into chlorophenols. Environ Chem Lett 17:1111–1116
Jiang C, Yu H, Lu Y, Zhu S, Geng Z, Huo M, Wang X (2018c) Preparation of spike-like palladium nanoparticle electrode and its dechlorination properties. Thin Solid Films 664:27–32
Jiang G, Wang K, Li J, Fu W, Zhang Z, Johnson G, Lv X, Zhang Y, Zhang S, Dong F (2018b) Electrocatalytic hydrodechlorination of 2, 4-dichlorophenol over palladium nanoparticles and its pH-mediated tug-of-war with hydrogen evolution. Chem Eng J 348:26–34
Jiang X, Chen Y, Hou C, Liu X, Ou C, Han W, Sun X, Li J, Wang L, Shen J (2018a) Promotion of para-chlorophenol reduction and extracellular electron transfer in an anaerobic system at the presence of iron-oxides. Front Microbiol 9:2052
Khataee AR, Fathinia M, Zarei M, Izadkhah B, Joo SW (2014) Modeling and optimization of photocatalytic/photoassisted-electro-Fenton like degradation of phenol using a neural network coupled with genetic algorithm. J Ind Eng Chem 20:1852–1860
Le X, Dong Z, Li X, Zhang W, Le M, Ma J (2015) Fibrous nano-silica supported palladium nanoparticles: an efficient catalyst for the reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol under mild conditions. Catal Commun 59:21–25
Lee CS, Oh DS, Le TT, Gong J, Chang YS (2018) Ligand-assisted sequential redox degradation of tetrabromobisphenol A using bimetallic zero-valent iron nanoparticles. Ind Eng Chem Res 57:17329–17337
Li J, Peng Y, Zhang W, Shi X, Chen M, Wang P, Zhang X, Fu H, Lv X, Dong F, Jiang G (2020) Hierarchical Pd/MnO2 nanosheet array supported on Ni foam: an advanced electrode for electrocatalytic hydrodechlorination reaction. Appl Surf Sci 509:145369
Li J, Wang H, Wang L, Ma C, Luan C, Zhao B, Zhang Z, Zhang H, Cheng X, Liu J (2018) The preparation of Pd/foam-Ni electrode and its electrocatalytic hydrodechlorination for monochlorophenol isomers. Catalysts 8:378
Li J, Xiong Z, Yu Y, Wang X, Zhou H, Huang B, Wu Z, Yu C, Chen T, Pan Z, Yao G, Lai B (2021) Efficient degradation of carbamazepine by electro-Fenton system without any extra oxidant in the presence of molybdate: the role of slow release of iron ions. Appl Catal B 298:120506
Li K, Yang Y, Bacha AUR, Feng Y, Ajmal S, Nabi I, Zhang L (2019) Efficiently complete degradation of 2, 4-DCP using sustainable photoelectrochemical reduction and sequential oxidation method. Chem Eng J 378:122191
Lin H, Zhang H, Wang X, Wang L, Wu J (2014) Electro-Fenton removal of Orange II in a divided cell: reaction mechanism, degradation pathway and toxicity evolution. Sep Purif Technol 122:533–540
Liu A, Shi Q, Fu Y, Tang B, Zhang J (2021) Electrocatalytic hydrodechlorination of 2, 4-dichlorophenol by Pd-MWCNTs-foam nickel electrode. Acta Sci Circumst 41(10):3976–3984 (in Chinese)
Liu Y, Mao R, Tong Y, Lan H, Zhang G, Liu H, Qu J (2017) Reductive dechlorination of trichloroacetic acid (TCAA) by electrochemical process over Pd-In/Al2O3 catalyst. Electrochimica Acta 232:13–21
Ma H, Zhang X, Ma Q, Wang B (2009) Electrochemical catalytic treatment of phenol wastewater. J Hazard Mater 165:475–480
Moreira FC, Boaventura RAR, Brillas E, Vilar VJP (2017) Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl Catal B 202:217–261
Moreira FC, Soler J, Fonseca A, Saraiva I, Boaventura RAR, Brillas E, Vilar VJP (2016) Electrochemical advanced oxidation processes for sanitary landfill leachate remediation: evaluation of operational variables. Appl Catal B 182:161–171
Oladipo AC, Tella AC, Clayton HS, Olayemi VT, Akpor OB, Dembaremba TO, Ogunlaja AS, Clarkson GJ, Walton RI (2022) A zinc-based coordination polymer as adsorbent for removal of trichlorophenol from aqueous solution: synthesis, sorption and DFT studies. J Mol Struct 1247:131274
Oputu OU, Fatoki OS, Opeolu BO, Akharame MO (2019) Degradation pathway of ozone oxidation of phenols and chlorophenols as followed by LC-MS-TOF. Ozone Sci Eng 42:294–318
Pimentel M, Oturan N, Dezotti M, Oturan MA (2008) Phenol degradation by advanced electrochemical oxidation process electro-Fenton using a carbon felt cathode. Appl Catal B 83:140–149
Shu X, Yang Q, Yao F, Zhong Y, Ren W, Chen F, Sun J, Ma Y, Fu Z, Wang D, Li X (2019) Electrocatalytic hydrodechlorination of 4-chlorophenol on Pd supported multi-walled carbon nanotubes particle electrodes. Chem Eng J 358:903–911
Sirés I, Garrido JA, Rodríguez RM, Brillas E, Oturan N, Oturan MA (2007) Catalytic behavior of the Fe3+/Fe2+ system in the electro-Fenton degradation of the antimicrobial chlorophene. Appl Catal B 72:382–394
Song G, Du X, Zheng Y, Su P, Tang Y, Zhou M (2022) A novel electro-Fenton process coupled with sulfite: enhanced Fe3+ reduction and TOC removal. J Hazard Mater 422:126888
Wan W, Zhang Y, Ji R, Wang B, He F (2017) Metal foam-based Fenton-like process by aeration. ACS Omega 2:6104–6111
Wang CT, Chou WL, Chung MH, Kuo YM (2010) COD removal from real dyeing wastewater by electro-Fenton technology using an activated carbon fiber cathode. Desalination 253:129–134
Wang YX, Cui YY, Zhang Y, Yang CX (2022) Synthesis of reusable and renewable microporous organic networks for the removal of halogenated contaminants. J Hazard Mater 424:127485
Wei X, Wan X, Sun Z, Miao J, Zhang R, Niu QJ (2018) Understanding electrocatalytic hydrodechlorination of chlorophenols on palladium-modified cathode in aqueous solution. ACS Omega 3:5876–5886
Wu C, Zhou L, Zhou C, Zhou Y, Xia S, Rittmann BE (2022) Co-removal of 2, 4-dichlorophenol and nitrate using a palladized biofilm: denitrification-promoted microbial mineralization following catalytic dechlorination. J Hazard Mater 422:126916
Xiao J, Xie Y, Han Q, Cao H, Wang Y, Nawaz F, Duan F (2016) Superoxide radical-mediated photocatalytic oxidation of phenolic compounds over Ag+/TiO2: influence of electron donating and withdrawing substituents. J Hazard Mater 304:126–133
Xu P, Xu H, Zheng D (2019) The efficiency and mechanism in a novel electro-Fenton process assisted by anodic photocatalysis on advanced treatment of coal gasification wastewater. Chem Eng J 361:968–974
Yang K, Zhao Y, Ji M, Li Z, Zhai S, Zhou X, Wang Q, Wang C, Liang B (2021) Challenges and opportunities for the biodegradation of chlorophenols: aerobic, anaerobic and bioelectrochemical processes. Water Res 193:116862
Yang L, Chen Z, Cui D, Luo X, Liang B, Yang L, Liu T, Wang A, Luo S (2019) Ultrafine palladium nanoparticles supported on 3D self-supported Ni foam for cathodic dechlorination of florfenicol. Chem Eng J 359:894–901
Yao F, Zhong Y, Yang Q, Wang D, Chen F, Zhao J, Xie T, Jiang C, An H, Zeng G, Li X (2017) Effective adsorption/electrocatalytic degradation of perchlorate using Pd/Pt supported on N-doped activated carbon fiber cathode. J Hazard Mater 323:602–610
Yazdanbakhsh A, Eslami A, Moussavi G, Rafiee M, Sheikhmohammadi A (2018) Photo-assisted degradation of 2, 4, 6-trichlorophenol by an advanced reduction process based on sulfite anion radical: degradation, dechlorination and mineralization. Chemosphere 191:156–165
Yu H, Yang S, Zhao B, Lu Y, Zhu S, Wang X, Qin W, Huo M (2020) Enhanced electrochemical dechlorination of 4-chlorophenol on a Ni-foam electrode modified with palladium, polypyrrole and graphene. J Electroanal Chem 869:114099
Zhang Y, Zhang G, Liu W, Li M, Jiang S, Hao L, Wang Z, Wu Q, Wang C (2022) Fabrication of carbonyl-functional hypercrosslinked polymers as solid-phase extraction sorbent for enrichment of chlorophenols from water, honey and beverage samples. Microchim Acta 189:21
Zhao H, Wang Y, Wang Y, Cao T, Zhao G (2012) Electro-Fenton oxidation of pesticides with a novel Fe3O4@Fe2O3/activated carbon aerogel cathode: high activity, wide pH range and catalytic mechanism. Appl Catal B 125:120–127
Zhou J, Lou Z, Yang K, Xu J, Li Y, Liu Y, Baig SA, Xu X (2019) Electrocatalytic dechlorination of 2, 4-dichlorobenzoic acid using different carbon-supported palladium moveable catalysts: adsorption and dechlorination activity. Appl Catal B 244:215–224
Zhou X, Luo H, Chen Q, Cao X, Shen C, Zhou J (2020) Catalytic hydrodechlorination and advanced oxidation processes of 2, 4-dichlorophenoxyacetic acid over CMK-3 supported catalyst: the bi-functional effect of metal Pd. Chem Eng J 402:126175
Funding
National Key Research and Development Program of China (No. 2018YFD0800601) and Chongqing Natural Science Foundation of China (cstc2021jcyj-msxmX1126).
Author information
Authors and Affiliations
Contributions
Weibin Huang: methodology, investigation, data curation, and writing — reviewing and editing. Andi Liu: conceptualization, methodology, investigation, and writing — reviewing and editing. Bobin Tang: methodology and investigation. Yuanhang Fu: methodology and investigation. Jinzhong Zhang: supervision, conceptualization, funding acquisition, and writing — reviewing and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, W., Liu, A., Tang, B. et al. Efficient degradation of 2,4-dichlorophenol in water by sequential electrocatalytic reduction and oxidation with a Pd-MWCNTs/Ni-foam electrode. Environ Sci Pollut Res 30, 70760–70770 (2023). https://doi.org/10.1007/s11356-023-27464-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27464-6