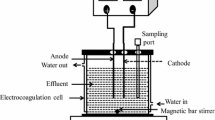
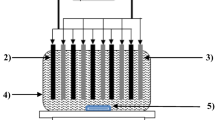
Abstract
The energy and electrode costs are the restrictions of applying electrocoagulation (EC) in wastewater treatment and many attempts have been made to decrease these costs. In this study, an economical EC was investigated to treat a hazardous anionic azo dye wastewater (DW) that threatens the environment and human health. Firstly, an electrode for EC process was produced from recycled aluminum cans (RACs) by remelting in an induction melting furnace. The performance of the RAC electrodes in the EC was evaluated for COD, color removal, and the EC operating parameters such as initial pH, current density (CD), and electrolysis time. Response surface methodology which is based on central composite design (RSM-CCD) was used for the optimization of the process parameters which were found to be pH 3.96, CD 15 mA/cm2, and electrolysis time 45 min. The maximum COD and color removal values were determined as 98.87% and 99.07%, respectively. The characterization of electrodes and the EC sludge was conducted by XRD, SEM, and EDS analyses for the optimum variables. In addition, the corrosion test was conducted to determine the theoretical lifetime of the electrodes. The results showed that the RAC electrodes show an extended lifetime as compared to their counterparts. Secondly, the energy cost required to treat DW in the EC was aimed to decrease by using solar panels (PV), and the optimum number of PV for the EC was determined by the MATLAB/Simulink. Consequently, the EC with low treatment cost was proposed for the treatment of DW. An economical and efficient EC process for waste management and energy policies was investigated in the present study which will be instrumental in the emergence of new understandings.










Similar content being viewed by others
Data availability
Data will be made available on request.
Abbreviations
- DC:
-
Direct current
- EC:
-
Electrocoagulation
- CD:
-
Current density
- Time:
-
Electrolysis time
- RSM :
-
Response surface methodology
- CCD:
-
Central composite design
- RAC:
-
Recycling aluminum cans
- Fe:
-
Iron
- Al:
-
Aluminum
- ANOVA:
-
The analysis of variance
- CR:
-
Congo Red
- COD:
-
Chemical oxygen demand
- SEM :
-
Scanning electron microscope
- FESEM:
-
Field-emission scanning electron microscope
- FTIR:
-
Fourier transform infrared spectroscopy analysis
- XRD:
-
X-ray diffraction
- EDS:
-
Electron dispersive spectroscopy
- OC :
-
Operating cost
- ENC:
-
Electrical energy consumption
- ELC:
-
Electrode consumption
- EC-F:
-
Electrocoagulation-flotation
- DW:
-
Dye-containing wastewater
References
Abdulrazzaq NN, Al-Sabbagh BH, Shanshool HA (2021) Coupling of electrocoagulation and microflotation for the removal of textile dyes from aqueous solutions. J Water Process Eng 40:101906. https://doi.org/10.1016/J.JWPE.2020.101906
Ahmad MA, Alrozi R (2010) Optimization of preparation conditions for mangosteen peel-based activated carbons for the removal of Remazol Brilliant Blue R using response surface methodology. Chem Eng J 165(3):883–890
Akhtar A, Aslam Z, Asghar A, Bello MM, Raman AAA (2020) Electrocoagulation of Congo Red dye-containing wastewater: optimization of operational parameters and process mechanism. J Environ Chem Eng 8(5):104055
Akkaya GK, Sekman E, Top S, Sagir E, Bilgili MS, Guvenc SY (2017) Enhancing filterability of activated sludge from landfill leachate treatment plant by applying electrical field ineffective on bacterial life. Environ Sci Pollut Res 24:10364–10372
Akkaya GK (2022) Treatment of petroleum wastewater by electrocoagulation using scrap perforated (Fe-anode) and plate (Al and Fe-cathode) metals: optimization of operating parameters by RSM. Chem Eng Res Des 187:261–275. https://doi.org/10.1016/J.CHERD.2022.08.048
Akyol A (2012) Treatment of paint manufacturing wastewater by electrocoagulation. Desalination 285:91–99
Al-Degs Y, Khraisheh MAM, Allen SJ, Ahmad MN (2000) Effect of carbon surface chemistry on the removal of reactive dyes from textile effluent. Water Res 34(3):927–935
Alaba PA, Sani YM, Mohammed IY, Abakr YA, Wan Daud WMA (2017) Synthesis of hierarchical nanoporous HY zeolites from activated kaolin, a central composite design optimization study. Adv Powder Technol 28(5):1399–1410. https://doi.org/10.1016/J.APT.2017.03.008
AlJaberi FY, Alardhi SM, Ahmed SA, Salman AD, Juzsakova T, Cretescu I, Le PC, Chung WJ, Chang SW, Nguyen DD (2022) Can electrocoagulation technology be integrated with wastewater treatment systems to improve treatment efficiency? Environ Res 214:113890. https://doi.org/10.1016/J.ENVRES.2022.113890
Al-Tohamy R, Ali SS, Li F, Okasha KM, Mahmoud YAG, Elsamahy T, ..., Sun J (2022) A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol Environ Saf 231:113160
AlSaffar KA, Bdeir LMH (2008) Recycling of aluminum beverage cans. J Eng Technol 12(3):157–163
Aoudj S, Khelifa A, Drouiche N, Hecini M, Hamitouche H (2010) Electrocoagulation process applied to wastewater containing dyes from textile industry. Chem Eng Process 49(11):1176–1182
Asghar A, Abdul Raman AA, Daud WW (2017) Sequential optimization for minimizing material cost and treatment time of fenton oxidation for textile wastewater treatment. Chem Eng Commun 204(8):873–883
Asghar A, Ramzan N, Jamal B, Maqsood M, Sajjadi B, Chen W (2020) Low frequency ultrasonic-assisted Fenton oxidation of textile wastewater: process optimization and electrical energy evaluation. Water Environ J 34(4):523–535
Aygün A, Nas B, Sevimli MF (2021) Electrocoagulation of disperse dyebath wastewater: optimization of process variables and sludge production. J of Electrochem Sci Technol 12(1):82–91
Bajpai S, Gupta SK, Dey A, Jha MK, Bajpai V, Joshi S, Gupta A (2012) Application of central composite design approach for removal of chromium (VI) from aqueous solution using weakly anionic resin: modeling, optimization, and study of interactive variables. J Hazard Mater 227:436–444
Bani-Melhem K, Al-Kilani MR, Tawalbeh M (2023) Evaluation of scrap metallic waste electrode materials for the application in electrocoagulation treatment of wastewater. Chemosphere 310:136668. https://doi.org/10.1016/J.CHEMOSPHERE.2022.136668
Bayar S, Yildiz YS, Yilmaz AE, Irdemez S (2011) The effect of stirring speed and current density on removal efficiency of poultry slaughterhouse wastewater by electrocoagulation method. Desalination 280(1–3):103–107. https://doi.org/10.1016/J.DESAL.2011.06.061
Behera SK, Meena H, Chakraborty S, Meikap BC (2018) Application of response surface methodology (RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int J Min Sci Technol 28(4):621–629. https://doi.org/10.1016/J.IJMST.2018.04.014
Bellini A, Bifaretti S, Iacovone V, Cornaro C (2009) Simplified model of a photovoltaic module. Applied Electronics, Rome
Bener S, Bulca Ö, Palas B, Tekin G, Atalay S, Ersöz G (2019) Electrocoagulation process for the treatment of real textile wastewater: effect of operative conditions on the organic carbon removal and kinetic study. Process Saf Environ Prot 129:47–54
Can-Güven E (2021) Advanced treatment of dye manufacturing wastewater by electrocoagulation and electro-Fenton processes: effect on COD fractions, energy consumption, and sludge analysis. J Environ Manag 300:113784. https://doi.org/10.1016/J.JENVMAN.2021.113784
Cardoso JC, Bessegato GG, BoldrinZanoni MV (2016) Efficiency comparison of ozonation, photolysis, photocatalysis and photoelectrocatalysis methods in real textile wastewater decolorization. Water Res 98:39–46. https://doi.org/10.1016/J.WATRES.2016.04.004
Costa AL, Gomes AC, Lopes AD, Da Silva JP, Pillinger M, Gonçalves IS, de Melo JSS (2020) Evaluation of the supramolecular interaction of Congo red with cucurbiturils using mass spectrometry and spectroscopic methods. New J Chem 44(6):2587–2596
Dalvand A, Gholami M, Joneidi A, Mahmoodi NM (2011) Dye removal, energy consumption and operating cost of electrocoagulation of textile wastewater as a clean process. Clean-Soil Air Water 39(7):665–672
Das PP, Sharma M, Purkait MK (2022) Recent progress on electrocoagulation process for wastewater treatment: a review. Separation Purif Technol 292:121058. https://doi.org/10.1016/J.SEPPUR.2022.121058
Elabbas S, Ouazzani N, Mandi L, Berrekhis F, Perdicakis M, Pontvianne S, Pons M-N, Lapicque F, Leclerc J-P (2016) Treatment of highly concentrated tannery wastewater using electrocoagulation: influence of the quality of aluminium used for the electrode. J Hazard Mater 319:69–77
Elazzouzi M, Haboubi K, Elyoubi MS, El Kasmi A (2021) Development of a novel electrocoagulation anode for real urban wastewater treatment: experimental and modeling study to optimize operative conditions. Arab J Chem 14(1):102912. https://doi.org/10.1016/J.ARABJC.2020.11.018
Elkacmi R, Boudouch O, Hasib A, Bouzaid M, Bennajah M (2020) Photovoltaic electrocoagulation treatment of olive mill wastewater using an external-loop airlift reactor. Sustain Chem Pharm 17(May):100274. https://doi.org/10.1016/j.scp.2020.100274
Emerick T, Vieira JL, Silveira MHL, João JJ (2020) Ultrasound-assisted electrocoagulation process applied to the treatment and reuse of swine slaughterhouse wastewater. J Environ Chem Eng 8(6):104308
Federation WE, Association A (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC, USA, p 21
Golder AK, Samanta AN, Ray S (2006) Anionic reactive dye removal from aqueous solution using a new adsorbent—sludge generated in removal of heavy metal by electrocoagulation. Chem Eng J 122(1–2):107–115. https://doi.org/10.1016/J.CEJ.2006.06.003
Goudjil S, Guergazi S, Masmoudi T, Achour S (2021) Effect of reactional parameters on the elimination of Congo Red by the combination of coagulation–floculation with aluminum sulfate. Desalin Water Treat 209:429–436
Huda N, Raman AAA, Bello MM, Ramesh S (2017) Electrocoagulation treatment of raw landfill leachate using iron-based electrodes: effects of process parameters and optimization. J Environ Manage 204:75–81
Hussin F, Abnisa F, Issabayeva G, Aroua MK (2017) Removal of lead by solar-photovoltaic electrocoagulation using novel perforated zinc electrode. J Clean Prod 147:206–216
Idusuyi N, Ajide OO, Abu R, Okewole OA, Ibiyemi OO (2022) Low cost electrocoagulation process for treatment of contaminated water using aluminium electrodes from recycled cans. Mater Today: Proc 56:1712–1716. https://doi.org/10.1016/J.MATPR.2021.10.352
Inayat M, Sulaiman SA, Kurnia JC (2019) Catalytic co-gasification of coconut shells and oil palm fronds blends in the presence of cement, dolomite, and limestone: parametric optimization via Box Behnken Design. J Energy Inst 92(4):871–882
Jiang C, Zhang J (2007) Progress and prospect in electro-Fenton process for wastewater treatment. J Zhejiang Univ-Sci A 8(7):1118–1125
Jin P, Zhu J, Yuan S, Zhang G, Volodine A, Tian M, Wang J, Luis P, Van der Bruggen B (2021) Erythritol-based polyester loose nanofiltration membrane with fast water transport for efficient dye/salt separation. Chem Eng J 406:126796. https://doi.org/10.1016/J.CEJ.2020.126796
Kamyab H, Yuzir MA, Al-Qaim FF, Purba LDA, Riyadi FA (2021) Application of Box-Behnken design to mineralization and color removal of palm oil mill effluent by electrocoagulation process. Environ Sci Pollut Res 1–13. https://doi.org/10.1007/s11356-021-16197-z
Kannan N, Vakeesan D (2016) Solar energy for future world:-a review. Renew Sustain Energy Rev 62:1092–1105
Khandegar V, Saroha AK (2012) Electrochemical treatment of distillery spent wash using aluminum and ıron electrodes. Chin J Chem Eng 20(3):439–443. https://doi.org/10.1016/S1004-9541(11)60204-8
Khemila B, Merzouk B, Chouder A, Zidelkhir R, Leclerc J-P, Lapicque F (2018) Removal of a textile dye using photovoltaic electrocoagulation. Sustain Chem Pharm 7:27–35
Khorram AG, Fallah N (2018) Treatment of textile dyeing factory wastewater by electrocoagulation with low sludge settling time: optimization of operating parameters by RSM. J Environ Chem Eng 6(1):635–642
Kim S-H, Choi P-P (2017) Enhanced Congo red dye removal from aqueous solutions using iron nanoparticles: adsorption, kinetics, and equilibrium studies. Dalton Trans 46(44):15470–15479
Kobya M, Demirbas E, Can OT, Bayramoglu M (2006) Treatment of levafix orange textile dye solution by electrocoagulation. J Hazard Mater 132(2–3):183–188
Kumar D, Sharma C (2022) Paper industry wastewater treatment by electrocoagulation and aspect of sludge management. J Clean Prod 360:131970
Lach CE, Pauli CS, Coan AS, Simionatto EL, Koslowski LAD (2022) Investigating the process of electrocoagulation in the removal of azo dye from synthetic textile effluents and the effects of acute toxicity on Daphnia magna test organisms. J Water Process Eng 45:102485
Litefti K, Freire MS, Stitou M, González-Álvarez J (2019) Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci Rep 9(1):1–11
Ma SS (2016) Electrolytic removal of alizarin red S by Fe/Al composite hydrogel electrode for electrocoagulation toward a new wastewater treatment. Environ Sci Pollut Res 23(22):22771–22782
Mittal A, Mittal J, Malviya A, Gupta VK (2009) Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J Colloid Interface Sci 340(1):16–26. https://doi.org/10.1016/J.JCIS.2009.08.019
Mohammadlo N, Rasoulifard MH, Vahedpour M, Eskandarian MR (2014) The kinetic and thermodynamic study for decolorization of Congo red from aqueous solution using electrocoagulation process. J Appl Chem Res 8(4):123–142
Moussa DT, El-Naas MH, Nasser M, Al-Marri MJ (2017) A comprehensive review of electrocoagulation for water treatment: potentials and challenges. J Environ Manage 186:24–41
Nandiyanto ABD, Oktiani R, Ragadhita R (2019) How to read and interpret FTIR spectroscope of organic material. Indonesian J Sci Technol 4(1):97–118
Negarestani M, Motamedi M, Kashtiaray A, Khadir A, Sillanpää M (2020) Simultaneous removal of acetaminophen and ibuprofen from underground water by an electrocoagulation unit: operational parameters and kinetics. Groundw Sustain Dev 11:100474
Nidheesh PV, Singh TSA (2017) Arsenic removal by electrocoagulation process: recent trends and removal mechanism. Chemosphere 181:418–432. https://doi.org/10.1016/j.chemosphere.2017.04.082
Nippatla N, Philip L (2020) Electrochemical process employing scrap metal waste as electrodes for dye removal. J Environ Manag 273:111039. https://doi.org/10.1016/J.JENVMAN.2020.111039
Oliveira JT, de Sousa MC, Martins IA, de Sena LMG, Nogueira TR, Vidal CB,..., do Nascimento RF (2021) Electrocoagulation/oxidation/flotation by direct pulsed current applied to the removal of antibiotics from Brazilian WWTP effluents. Electrochimica Acta 388:138499
Otitoju TA, Ouyang Y, Jiang D, Shoparwe NF, Jansen JC, Wang S, Zhang A, Sun T, Li S (2022) Efficient removal of chemical oxygen demand from lye wastewater by APTES-TIO2/GO mixed matrix membrane: optimization using Box-Behnken Design. J Clean Prod 336:130379. https://doi.org/10.1016/J.JCLEPRO.2022.130379
Palanisamy S, Nachimuthu P, Awasthi MK, Ravindran B, Chang SW, Palanichamy M, Nguyen DD (2020) Application of electrochemical treatment for the removal of triazine dye using aluminium electrodes. J Water Supply Res Technol AQUA 69(4):345–354
Pathak A, Khandegar V, Kumar A (2021) Statistical ınvestigation in conjunction with a Box-Behnken design for the removal of dyes using electrocoagulation. J Hazard Toxic Radioact Waste 26(2):4022001
Pi Y, Duan C, Zhou Y, Sun S, Yin Z, Zhang H, Liu C, Zhao Y (2022) The effective removal of Congo Red using a bio-nanocluster: Fe3O4 nanoclusters modified bacteria. J Hazard Mater 424:127577. https://doi.org/10.1016/J.JHAZMAT.2021.127577
Pigorsch E, Elhaddaoui A, Turrell S (1994) Spectroscopic study of pH and solvent effects on the structure of Congo red and its binding mechanism to amyloid-like proteins. Spectrochim Acta Part A 50(12):2145–2152. https://doi.org/10.1016/0584-8539(94)00151-0
Sankar MSR, Sivasubramanian V, Vijay EVV, Jerold M, Kanimozhi J, Sinu P, Shankar N (2018) Kinetic, isothermal and thermodynamic investigation on electrocoagulation of Congo red dye removal from synthetic wastewater using aluminium electrodes. Desalin Water Treat 122:339–350
Sankar R, Sivasubramanian V (2020) Application of statistical design to optimize the electrocoagulation of synthetic Congo red dye solution and predicting the mechanism. Int J Environ Sci Technol 17(3):1373–1386
Sankar MR, Sivasubramanian V (2021) Optimization and evaluation of malathion removal by electrocoagulation process and sludge management. J Environ Chem Eng 9(5):106147
Sismanoglu T, Pura S, Bastug AS (2006) Binary and ternary metal complexes of Congo red with amino acids. Dyes and Pigments 70(2):136–142
Song D, Kadier A, Peralta-Hernández JM, Xie H, Hao B, Ma PC (2022) Separation of oil-water emulsions by a novel packed bed electrocoagulation (EC) process using anode from recycled aluminum beverage cans. J Clean Prod 379:134693. https://doi.org/10.1016/J.JCLEPRO.2022.134693
Suresh A, Sathish S, Narendrakumar G (2019) Electrocoagulation of azo dye containing synthetic wastewater using monopolar iron electrodes and the characterization of the sludge. Water Practice Technol 14(3):587–597
Valero D, Ortiz JM, Exposito E, Montiel V, Aldaz A (2008) Electrocoagulation of a synthetic textile effluent powered by photovoltaic energy without batteries: direct connection behaviour. Sol Energy Mater Sol Cells 92(3):291–297
Vidal J, Villegas L, Peralta-Hernández JM, Salazar González R (2016) Removal of Acid Black 194 dye from water by electrocoagulation with aluminum anode. J Environ Sci Health Part A 51(4):289–296
Vignesh A, Siddarth A, Babu BR (2017) Electro-dissolution of metal scrap anodes for nickel ion removal from metal finishing effluent. J Mater Cycles Waste Manage 19(1):155–162
Yasasve M, Manjusha M, Manojj D, Hariharan NM, Sai Preethi P, Asaithambi P, Karmegam N, Saravanan M (2022) Unravelling the emerging carcinogenic contaminants from industrial waste water for prospective remediation by electrocoagulation – a review. Chemosphere 307:136017. https://doi.org/10.1016/J.CHEMOSPHERE.2022.136017
Acknowledgements
In the study, a copper mold manufactured in the NEU-BAP project numbered 211219005 was used to obtain RAC electrodes. Some portions of information contained in this publication are printed with the permission of Minitab, LLC. All such material remains the exclusive property and copyright of Minitab, LLC. All rights reserved.
Author information
Authors and Affiliations
Contributions
Gülizar Kurtoğlu Akkaya: conceptualization; methodology; investigation; data curation; writing—original draft; writing—review and editing; visualization; project administration. Gökhan Polat: conceptualization; methodology; investigation; writing—original draft; writing—review and editing, visualization. Gamze Nalçacı: methodology; investigation; writing—original draft; writing—review and editing; visualization. Yasin Ramazan Eker: conceptualization, investigation, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
This research did not contain any studies involving animal or human participants, nor did it take place on any private or protected areas.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme L. Dotto
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akkaya, G.K., Polat, G., Nalçacı, G. et al. An economical electrocoagulation process of a hazardous anionic azo dye wastewater with the combination of recycled electrodes and solar energy. Environ Sci Pollut Res 30, 70331–70347 (2023). https://doi.org/10.1007/s11356-023-27375-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27375-6