Abstract
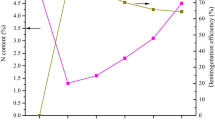
Urea–formaldehyde (UF) resin is difficult to degrade and classified as hazardous organic waste. To address this concern, the co-pyrolysis behavior of UF resin with pine sawdust (PS) was studied, and the adsorption properties of pyrocarbon were evaluated with Cr (VI). Thermogravimetric analysis revealed that adding a small amount of PS can improve the pyrolysis behavior of UF resin. Based on the Flynn Wall Ozawa (FWO) method, the kinetics and activation energy values were estimated. It was observed that when the amount of UF resin exceeded twice that of PS, the activation energy of the reaction decreased, and they acted synergistically. The characterization of pyrocarbon samples showed that the specific surface area increased with the increase of temperature, while the content of functional groups showed the opposite trend. Intermittent adsorption experiments showed that 5UF + PS400 achieved 95% removal of 50 mg/L Cr (VI) at 0.6 g/L dosage and at pH 2. The adsorption process was consistent with the Langmuir isotherm and pseudo-second-order kinetics, and the maximum adsorption was 143.66 mg/g at 30 ℃. Furthermore, the adsorption process consisted of electrostatic adsorption, chelation, and redox reaction. Overall, this study provides a useful reference for the co-pyrolysis of UF resin and the adsorption capacity of pyrocarbon.











Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Aydın YA, Aksoy ND (2009) Adsorption of chromium on chitosan: optimization, kinetics and thermodynamics. Chem Eng J 151:188–194
Cesprini E, Resente G, Causin V, Urso T, Cavalli R, Zanetti M (2020) Energy recovery of glued wood waste – a review. Fuel 262:116520
Chen Y, de Oliveira LM, da Silva EB (2017) Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere: Environmental toxicology and risk assessment 178:466–478
Chen K, Cheng X, Chen Y, Qi J, Xie J, Huang X, Jiang Y, Xiao H (2021) Thermal degradation kinetics of urea–formaldehyde resins modified by almond shells. ACS Omega 6:25702–25709
Feng YS, Chen SH, Mu J (2010) Characterization of products from pyrolysis of waste wood-based composites containing urea-formaldehyde resins. Adv Mater Res 139–141:185–189
Gao L, Li Z, Yi W, Li Y, Zhang P, Zhang A, Wang L (2021) Impacts of pyrolysis temperature on lead adsorption by cotton stalk-derived biochar and related mechanisms. J Environ Chem Eng 9:105602
Girods P, Rogaume Y, Dufour A, Rogaume C, Zoulalian A (2008) Low-temperature pyrolysis of wood waste containing urea–formaldehyde resin. Renew Energy 33:648–654
Guo J, Ren X, Li S, Huang Z, Manzo M, Cai L, Chang J (2021) The impact of blending with poplar wood on the co-pyrolysis characteristics of waste particleboards [J]. Biomass Conversion and Biorefinery 13(6):4949–4956
Hasan SH, Singh KK, Prakash O, Talat M, Ho YS (2008) Removal of Cr (VI) from aqueous solutions using agricultural waste ‘maize bran.’ J Hazard Mater 152:356–365
Hu S, Jess A, Xu M (2007) Kinetic study of Chinese biomass slow pyrolysis: comparison of different kinetic models. Fuel 86:2778–2788
Jain M, Garg VK, Kadirvelu K (2010) Adsorption of hexavalent chromium from aqueous medium onto carbonaceous adsorbents prepared from waste biomass. J Environ Manage 91:949–957
Jiang X, Li C, Yong C, Yan J (2010) TG-FTIR study on urea-formaldehyde resin residue during pyrolysis and combustion. J Hazard Mater 173:205–210
Kumar S, Narayanasamy S, Venkatesh RP (2018) Removal of cr(vi) from synthetic solutions using water caltrop shell as a low-cost biosorbent. Sep Sci Technol 1–17
Kumar S, Shahnaz T, Selvaraju N, Rajaraman PV (2020) Kinetic and thermodynamic studies on biosorption of Cr(VI) on raw and chemically modified Datura stramonium fruit. Environmental Monitoring and Assessment 192
Levankumar L, Muthukumaran V, Gobinath MB (2009) Batch adsorption and kinetics of chromium (VI) removal from aqueous solutions by Ocimum americanum L. seed pods. J Hazard Mater 161:709–713
Li C-Z (2013) Importance of volatile–char interactions during the pyrolysis and gasification of low-rank fuels – a review. Fuel 112:609–623
Li XT, Bing HL, Luo SY, Zhang WW, Zuo ZL, Ren DD (2022) Study on the pyrolysis behaviors of urea-formaldehyde resin and rice straw mixed pellets. Frontiers in Energy Research 9:813114
Liu J, Yang X, Liu H, Cheng W, Bao Y (2020) Modification of calcium-rich biochar by loading Si/Mn binary oxide after NaOH activation and its adsorption mechanisms for removal of Cu(II) from aqueous solution. Colloids Surf A: Physicochemical and Engineering Aspects 601:124960
Liu J, Yang XY, Liu HH, Jia XP, Bao YC (2021) Mixed biochar obtained by the co-pyrolysis of shrimp shell with corn straw: co-pyrolysis characteristics and its adsorption capability. Chemosphere 282
Liu M, Wang Y, Wu Y, Wan H (2018) Hydrolysis and recycling of urea formaldehyde resin residues. J Hazard Mater 355:96–103
Lubis MAR, Park B-D (2018) Analysis of the hydrolysates from cured and uncured urea-formaldehyde (UF) resins with two F/U mole ratios. Holzforschung 72:759–768
Manjuladevi M, Anitha R, Manonmani S (2018) Kinetic study on adsorption of Cr (VI), Ni(II), Cd(II) and Pb(II) ions from aqueous solutions using activated carbon prepared from Cucumis melo peel. Appl Water Sci 8:36
Mehr MR, Fekri MH, Omidali F, Eftekhari N, Akbari-Adergani B (2019) Removal of chromium (VI) from wastewater by palm kernel shell-based on a green method
Moussavi G, Barikbin B (2010) Biosorption of chromium(VI) from industrial wastewater onto pistachio hull waste biomass. Chem Eng J 162:893–900
Müsellim E, Tahir MH, Ahmad MS, Ceylan S (2018) Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl Therm Eng 137:54–61
Pan X, Gu Z, Chen W, Li Q (2021) Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: a review. Sci Total Environ 754:142104
Piekarski CM, de Francisco AC, da Luz LM, Kovaleski JL, Silva DAL (2017) Life cycle assessment of medium-density fiberboard (MDF) manufacturing process in Brazil. Sci Total Environ 575:103–111
Rai MK, Giri BS, Nath Y, Bajaj H, Soni S, Singh RP, Singh RS, Rai BN (2018) Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from almond shell: kinetics, equilibrium and thermodynamics study. J Water Supply Res Technol AQUA 67:724–737
Ren T, Wang Y, Wu N, Qing Y, Li X, Wu Y, Liu M (2022) Degradation of urea-formaldehyde resin residues by a hydrothermal oxidation method into recyclable small molecular organics. J Hazard Mater 426:127783
Roumeli E, Papadopoulou E, Pavlidou E, Vourlias G, Bikiaris D, Paraskevopoulos KM, Chrissafis K (2012) Synthesis, characterization and thermal analysis of urea–formaldehyde/nanoSiO2 resins. Thermochim Acta 527:33–39
Sun C, Li C, Tan H, Zhang Y (2019) Synergistic effects of wood fiber and polylactic acid during co-pyrolysis using TG-FTIR-MS and Py-GC/MS. Energy Convers Manage 202:112212
Vanreppelen K, Kuppens T, Thewys T, Carleer R, Yperman J, Schreurs S (2011) Activated carbon from co-pyrolysis of particle board and melamine (urea) formaldehyde resin: a techno-economic evaluation. Chem Eng J 172:835–846
Vyas A, Chellappa T, Goldfarb JL (2017) Porosity development and reactivity changes of coal–biomass blends during co-pyrolysis at various temperatures. J Anal Appl Pyrol 124:79–88
Wang H, Yuan X, Wu Y, Zeng G, Chen X, Leng L, Wu Z, Jiang L, Li H (2015) Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photocatalytic activity for Cr (VI) reduction. J Hazard Mater 286:187–194
Wang B, Xu F, Zong P, Zhang J, Tian Y, Qiao Y (2019) Effects of heating rate on fast pyrolysis behavior and product distribution of Jerusalem artichoke stalk by using TG-FTIR and Py-GC/ MS. Renew Energy 132:486–496
Wang G, Dai Y, Yang H, Xiong Q, Wang K, Zhou (2020) A review of recent advances in biomass pyrolysis. Energy And Fuels 34:15557–15578
Xiao YL, Xue YW, Gao F, Mosa A (2017) Sorption of heavy metal ions onto crayfish shell biochar: effect of pyrolysis temperature, pH and ionic strength. Journal of the Taiwan Institute of Chemical Engineers 80:114–121
Yuan J-H, Xu R-K, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Biores Technol 102:3488–3497
Yuan R, Yu S, Shen Y (2019) Pyrolysis and combustion kinetics of lignocellulosic biomass pellets with calcium-rich wastes from agro-forestry residues. Waste Manage 87:86–96
Zhang C, Zhang Z, Zhang L, Li Q, Li C, Chen G, Zhang S, Liu Q, Hu X (2020a) Evolution of the functionalities and structures of biochar in pyrolysis of poplar in a wide temperature range. Biores Technol 304:123002
Zhang J, Jin J, Wang M, Naidu R, Liu Y, Man YB, Liang X, Wong MH, Christie P, Zhang Y, Song C, Shan S (2020b) Co-pyrolysis of sewage sludge and rice husk/ bamboo sawdust for biochar with high aromaticity and low metal mobility. Environ Res 191:110034
Zhang ZY, Gao TT, Si SX, Liu QZ, Wu Y, Zhou GW (2018) One-pot preparation of P(TA-TEPA)-PAM-RGO ternary composite for high efficient Cr(VI) removal from aqueous solution. Chemical Engineering Journal 343:207–216
Zhao T-T, Ge W-Z, Nie Y-X, Wang Y-X, Zeng F-G, Qiao Y (2016) Highly efficient detoxification of Cr (VI) by brown coal and kerogen: process and structure studies. Fuel Process Technol 150:71–77
Zhong R, Gu J, Gao Z, Tu D, Hu C (2017) Impacts of urea-formaldehyde resin residue on recycling and reconstitution of wood-based panels. Int J Adhes Adhes 78:60–66
Zhou JG, Wang YF, Wang JT, Qiao WM, Long DH, Ling LC (2016) Effective removal of hexavalent chromium from aqueous solutions by adsorption on mesoporous carbon microspheres. Journal of colloid and interface science 462:200–207
Acknowledgements
This work was supported by the 2020 Science and Technology Project of Qingdao West Coast New Area (2020-99).
Author information
Authors and Affiliations
Contributions
Wanzhen Zhong: conceptualization, methodology, investigation, and writing—original draft. Xiaoteng Li: supervision and project administration. Siyi Luo: data curation and visualization. Weiqiang Tan: Software and resources. Zongliang Zuo: writing—review and editing. Dongdong Ren: validation and formal analysis.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Zhihong Xu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, W., Li, X., Luo, S. et al. The co-pyrolysis of waste urea–formaldehyde resin with pine sawdust: co-pyrolysis behavior, pyrocarbon and its adsorption performance for Cr (VI). Environ Sci Pollut Res 30, 72854–72866 (2023). https://doi.org/10.1007/s11356-023-27297-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27297-3