Abstract
For the first time, copper oxide–coated glass beads (CuO-GBs) were fabricated using physical vapor deposition (PVD) technology for sequestrating Pb2+ ions from solution is addressed. Compared to other coating procedures, PVD offered high-stability uniform CuO nano-layers attached with 3.0-mm glass beads. Heating of copper oxide–coated glass beads after deposition was rather necessary to achieve the best stability of the nano-adsorbent. Detection of nano-size copper oxide on the beads was made by FTIR (intense peak at 655 cm−1 for CuO bond stretching) and XRF (Cu peak at 8.0 keV). Scanning electron micrographs taken at high magnification power indicated the presence of CuO in nano-range deposited over glass beads. The maximum deposited amount of CuO on the beads was 1.1% and accomplished at the following operational conditions: internal pressure 10–5 mmHg, Ar flow rate 8.0 mL/min, voltage 84 V, pre-sputtering time 20 s, total sputtering time 10.0 min, and post-heating temperature 150 °C for 3 h. A univariate analysis indicated that the optimum Pb2+ uptake by CuO-GBs from solution was achieved at pH 7.0–8.0, 7 beads/50 mL, 120-min contact time, and 15-mg/L initial concentration. Kinetic data for Pb2+ uptake was best presented by a pseudo–second-order model with a relative prediction error of 3.2 and 5.1% for GBs and CuO-GBs, respectively. On the other hand, Pb2+ equilibrium isotherms at 25 °C were fairly presented by the Langmuir model, and the predicted saturation values were 5.48 and 15.69 mg/g for GBs and CuO-GBs, respectively. CuO and CuO-GBs had similar Pb2+ saturation values (~ 16 mg/g), although the latter demonstrated 4 times faster kinetic, thanks to fixation CuO on glass beads. Moreover, the chemical stability of copper oxide–coated glass beads was tested under different conditions. Recycling of copper oxide–coated glass beads was also investigated, and 90% of the surface was recovered using 0.01-M HNO3.
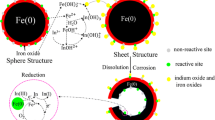
Graphical abstract








Similar content being viewed by others
Data availability
Data are available from the corresponding author upon request.
References
Abdelghani J, Khalili I, Al-Degs S, Abu-Nameh S (2019) Studying competitive retention of phthalate esters by humic acid under multi-variable experimental design optimization: interaction between experimental factors. Desalin Water Treat 150:320–330
Abdelghani J, Freihat R, El-Sheikh A (2020) Magnetic solid phase extraction of phthalate products from bottled, injectable and tap waters using graphene oxide: effect of oxidation method of graphene. J Environ Chem Eng 8:103527
Abu-Surrah A, Al-Degs Y (2022) Utilization of nanosize spent oil shale for water treatment: application of top-down nanonization technology for solid residues. Environ Sci Pollut Res 29:78314–78329
Al-degs Y, Khraisheh M, Tutunji M (2001) Sorption of lead ions on diatomite and manganese oxides modified diatomite. Water Res 35:3724–3728
Al-Ghouti M, Daana D (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:e122383
Al-Ghouti M, Ayman I, Bety A, Anas A, Al-Degs Y (2016) Multivariate analysis of competitive adsorption of food dyes by activated pine wood. Desalin Water Treat 57:27651–27662
Al-Zawahreh K, Barral M, Al-Degs Y, Paradelo R (2022a) Competitive removal of textile dyes from solution by pine bark-compost in batch and fixed bed column experiments. Environ Technol Innov 27:102421
Al-Zawahreh K, Al-Degs Y, Teresa M, Paradelo R (2022b) Optimization of direct blue 71 sorption by organic rich-compost following multilevel multifactor experimental design. Arab J Chem 15:103468
Andersen H, Siegrist H, Halling-Sørensen B, Ternes A (2003) Fate of estrogens in a municipal sewage treatment plant. Environ Sci Technol 37(18):4021–4026
Apul G, Wang Q, Zhou Y, Karanfil T (2013) Adsorption of aromatic organic contaminants by graphene nanosheets: comparison with carbon nanotubes and activated carbon. Water Res 47(4):1648–1654
Benjamin M, Sletten R, Bailey R, Bennett T (1996) sorption and filtration of metals using iron-oxide-coated sand. War Res 30(11):2609–2620
Cao J, Qin C, Wang Y, Zhang H, Zhang B, Gong Y, Wang X, Sunc G, Bala H, Zhang Z (2017) Synthesis of g-C3N4 nanosheet modified SnO2 composites with improved performance for ethanol gas sensing. RSC Adv 7:25504–25511
Dev VV, Nair KK, Baburaj G, Krishnan KA (2021) Monitoring of heavy metal contamination in Netravati river basin: overview of pollution indices and risk assessment. Sustain Water Resour Manag 7:20
Dev VV, Nair KK, Baburaj G, Krishnan KA (2022) Pushing the boundaries of heavy metal adsorption: a commentary on strategies to improve adsorption efficiency and modulate process mechanisms. Colloid Interf Sci Commun 49:100626
Dhoble M, Lunge S, Bhole A, Rayalu S (2011) Magnetic binary oxide particles (MBOP): a promising adsorbent for removal of As(III) in water. Water Res 45:4769–4781
Dil A, Ghaedi M, Asfaram A (2017) The performance of nanorods material as adsorbent for removal of azo dyes and heavy metal ions: application of ultrasound wave, optimization and modeling. Ultrason Sonochem 34:792–802
El-Sheikh A, Fasfous I, Al-Salamin R, Newman A (2018) Immobilization of citric acid and magnetite on sawdust for competitive adsorption and extraction of metal ions from environmental waters. J Environ Chem Eng 6:5186–5195
El-Sheikh A, Rania Q, Abdelghani J (2019) Adsorption and magnetic solid-phase extraction of NSAIDs from pharmaceutical wastewater using magnetic carbon nanotubes: effect of sorbent dimensions, magnetite loading and competitive adsorption study. Environ Technol Innov 16:100496
Fasfous I, El-Sheikh A, Awwad A, Al-Degs Y, Dawoud J (2021) Interaction influence of contact time and pH on cobalt retention by carbon nanotubes bearing various loads of TiO2 and Fe3O4. Curr Anal Chem 18:483–494
Graff N, Djurdianovic D (2022) Modelling, simulation and control of roll-to-roll physical vapor deposition processes. Procedia CIRP 113:546–551
Guedes M, Ferreira J, Ferro A (2009) A study on the aqueous dispersion mechanism of CuO powders using Tiron. J Colloid Interface Sci 330:119–124
Gupta K, Tyagi I, Agarwal S, Sadegh H, Shahryari-ghoshekandi R, Yari M, Yousefi-nejat O (2015) Experimental study of surfaces of hydrogel polymers HEMA, HEMA– EEMA–MA, and PVA as adsorbent for removal of azo dyes from liquid phase. J Mol Liq 206:129–136
Gupta V, Moradi O, Tyagi I, Agarwal S, Sadegh H, Shahryari-Ghoshekandi R, Makhlouf A, Goodarzi M, Garshasbi A (2016) Study on the removal of heavy metal ions from industry waste by carbon nanotubes: effect of the surface modification: a review. Crit Rev Environ Sci Technol 46(2):93–118
Ho Y, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Ijaz F, Shahid S, Khan S, Ahmad W, Zaman S (2017) Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: antimicrobial, antioxidant and photocatalytic dye degradation activities. Trop J Pharmaceu Res 16:743–753
Islam S, Choi S, Nam B, Yoon C, Lee H (2017) Needlelike iron oxide-CaCO3 adsorbents for ultrafast removal of anionic and cationic heavy metal ions. Chem Eng J 307:208–219
Khlifi R, Hamza-Chaffai A (2010) Head and neck cancer due to heavy metal exposure via tobacco smoking and professional exposure: a review. Toxicol Appl Pharmacol 248(2):71–88
Mazurkow J, Yüzbasi N, Domagala K, Pfeiffer S, Kata D, Graule T (2020) Nano-sized copper (oxide) on alumina granules for water filtration: effect of copper oxidation state on virus removal performance. Environ Sci Technol 54(2):1214–1222
Miao S, Bishay F, Chen M, Metcalfe D (2004) Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environ Sci Technol 38(13):3533–3541
Mishra P (2014) Adsorption–desorption of heavy metal ions. Curr Sci 107:601–612
Mona E, Ossman A, Marwa A (2015) CuO nanopowder for removal of Pb(II) and Zn(II). J Environ Eng Sci 10(1):10–18
Rickerby D, Morrison M (2007) Nanotechnology and the environment: a European perspective. Sci Technol Adv Mater 8(1):19–24
Sadegh H, Ali GAM, Gupta VK, Makhlouf ASH, Shahryarighoshekandi R, Nadagouda MN, Sillanpää M, Megiel E (2017) The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J Nanostructure Chem 7:1–14
Sadegh H, Ghoshekandi S, Masjedi A, Mahmoodi Z, Kazemi M (2016a) A review on carbon nanotubes adsorbents for the removal of pollutants from aqueous solutions. Int J Nano Dimens 7(2):109
Sadegh H, Zare K, Maazinejad B, Shahryari-Ghoshekandi R, Tyagi I, Agarwal S, Gupta K (2016b) Synthesis of MWCNTCOOH-cysteamine composite and its application for dye removal. J Mol Liq 215:221–228
Saha N, Rahman S, Ahmed B, Zhou L, Ngo H, Guo W (2017) Industrial metal pollution in water and probabilistic assessment of human health risk. J Environ Manag 185:70–78
Torres-Carrascoa M, Palomob J, Puertasa F (2014) Sodium silicate solutions from dissolution of glass wastes. Statistical analysis. Mater de Construcción 64:1–14
Vidyala S, Asghar W, Iqbal S (2011) Porous organic nanolayers for coating of solid state devices. J Nanobiotechnol 9:18
Wang S, Gong W, Liu X, Yao Y, Gao B, Yue Q (2007) Removal of lead(II) from aqueous solution by adsorption onto manganese oxide-coated carbon nanotubes. Sep Purif Technol 58:17–23
Zhao Y, Shen Y, Pan D, Hu Q, Xia H (2010) Preparation and characterization of amino-functionalized nano- Fe3O4 magnetic polymer adsorbents for removal of chromium (VI) ions. J Mater Sci 45(19):5291–5301
Acknowledgements
Dr. Abdelghani expresses gratitude to the Hashemite University Deanship of Scientific Research for providing excellent support for carrying out this research and for funding the procurement of PVD. We also like to thank all the technicians and research assistants who helped us conduct real-world experiments.
Author information
Authors and Affiliations
Contributions
Jafar I.M. Abdelghani: conceptualization, supervision, visualization, methodology, formal analysis, validation, investigation, writing—review and editing. Amjad H. El-Sheikh: experimental, analyzing data, writing—review and editing. Nabil N.AL-Hashimi: experimental, analyzing data, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme L. Dotto
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdelghani, J.I., El-Sheikh, A.H. & AL-Hashimi, N.N. Application of physical vapor deposition technology for practical utilization of nano-size copper oxide for lead uptake from solution: kinetics, equilibrium, and recycling studies. Environ Sci Pollut Res 30, 58783–58795 (2023). https://doi.org/10.1007/s11356-023-26591-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26591-4