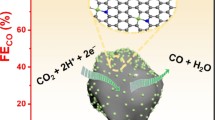
Abstract
The electrocatalytic reduction of CO2 towards CO is one of the most desirable routines to reduce atmospheric CO2 concentration and maintain a global carbon balance. In this work, a novel porous NiCu-embedded ZIF-derived N-doped carbon nanoparticle (NiCu@NCNPs) catalyst has been identified as an active, highly selective, stable, and cost-effective catalyst in CO2 reduction. A CO selectivity as high as 100% has been achieved on NiCu@NCNPs which is the highest reported to date. The particle current density of CO on NiCu@NCNPs is around 15 mA cm–2 under the optimized potential at −0.9 V vs. RHE. The NiCu@NCNPs electrode also exhibits excellent stability during the five sequential CO2 electroreduction experiments. The superior catalytic performance of NiCu@NCNPs in CO2RR can be related to its microstructure with high electrochemical surface area and low electron transfer resistance. Furthermore, a kinetic analysis has shown the formation of intermediate *COOH is the rate-determining step in CO2RR towards CO. According to the results of density functional theory (DFT) calculations, a low Gibbs-free energy change (∆G) for the rate-determining step leads to the enhanced catalytic performance of CO2RR on NiCu@NCNPs.








Similar content being viewed by others
Data availability
Data obtained and analyzed in this study are included in this article and available on reasonable request from the corresponding author.
References
Abbas M, Zhang Y, Koura YH et al (2022) The dynamics of renewable energy diffusion considering adoption delay. Sustain Prod Consum 30:387–395
Al-Rowaili FN, Jamal A, Ba Shammakh MS et al (2018) A review on recent advances for electrochemical reduction of carbon dioxide to methanol using metal–organic framework (MOF) and non-MOF catalysts: challenges and future prospects. ACS Sustainable Chem Eng 6(12):15895–15914
Chen Y, Ji S, Wang Y et al (2017) Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew Chem 129(24):7041–7045
Cheng J, Yang X, Xuan X et al (2020) Efficient conversion of carbon dioxide on atomically dispersed metal–Nx species-anchored porous carbon with embedded CuxCoy nanoparticles by accelerating electron separation. ACS Sustainable Chem Eng 8(15):5994–6002
Cui H, Guo Y, Guo L et al (2018) Heteroatom-doped carbon materials and their composites as electrocatalysts for CO2 reduction. J Mater Chem A 6(39):18782–18793
Davis SJ, Caldeira K, Matthews HD (2010) Future CO2 emissions and climate change from existing energy infrastructure. Science 329(5997):1330–1333
Duan X, Xu J, Wei Z et al (2017) Metal-free carbon materials for CO2 electrochemical reduction. Adv Mater 29(41):1701784
Gong S, Wang W, Xiao X et al (2021) Elucidating influence of the existence formation of anchored cobalt phthalocyanine on electrocatalytic CO2-to-CO conversion. Nano Energy 84:105904
Hu C, Bai S, Gao L et al (2019) Porosity-induced high selectivity for CO2 electroreduction to CO on Fe-doped ZIF-derived carbon catalysts. ACS Catal 9(12):11579–11588
Huang H, Jia H, Liu Z et al (2017) Understanding of strain effects in the electrochemical reduction of CO2: using Pd nanostructures as an ideal platform. Angew Chem Int Ed 129(13):3648–3652
Iqbal W, Tang YM, Chau KY et al (2021) Nexus between air pollution and NCOV-2019 in China: application of negative binomial regression analysis. Process Saf Environ 150:557–565
Jiang X, Li H, Xiao J et al (2018) Carbon dioxide electroreduction over imidazolate ligands coordinated with Zn (II) center in ZIFs. Nano Energy 52:345–350
Kaneco S, Iiba K, Ohta K et al (1998) Electrochemical reduction of CO2 on Au in KOH+ methanol at low temperature. J Electroanal Chem 441(1–2):215–220
Kattel S, Liu P, Chen JG (2017) Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface. J Am Chem Soc 139(29):9739–9754
Kitchin JR, Nørskov JK, Barteau MA et al (2004) Role of strain and ligand effects in the modification of the electronic and chemical properties of bimetallic surfaces. Phys Rev Lett 93(15):156801
Li J, Zitolo A, Garcés-Pineda FA et al (2021) Metal oxide clusters on nitrogen-doped carbon are highly selective for CO2 electroreduction to CO. ACS Catal 11(15):10028–10042
Liu S, Tao H, Zeng L et al (2017) Shape-dependent electrocatalytic reduction of CO2 to CO on triangular silver nanoplates. J Am Chem Soc 139(6):2160–2163
Liu S, Yang H, Su X et al (2019) Rational design of carbon-based metal-free catalysts for electrochemical carbon dioxide reduction: a review. J Energy Chem 36:95–105
Liu S, Yang H, Huang X et al (2018) Identifying active sites of nitrogen-doped carbon materials for the CO2 reduction reaction. Adv Funct Mater 28(21):1800499
Liu S, Yang HB, Hung SF et al (2020a) Elucidating the electrocatalytic CO2 reduction reaction over a model single-atom nickel catalyst. Angew Chem Int Ed 59(2):798–803
Liu Y, Tian D, Biswas AN et al (2020b) Transition metal nitrides as promising catalyst supports for tuning CO/H2 syngas production from electrochemical CO2 reduction. Angew Chem Int Ed 59(28):11345–11348
Liu Z, Tang YM, Chau KY et al (2021) Incorporating strategic petroleum reserve and welfare losses: a way forward for the policy development of crude oil resources in South Asia. Resour Policy 74:102309
Lu Q, Rosen J, Zhou Y et al (2014) A selective and efficient electrocatalyst for carbon dioxide reduction. Nat Commun 5(1):1–6
Nian Y, Wang Y, Biswas AN et al (2021) Trends and descriptors for tuning CO2 electroreduction to synthesis gas over Ag and Au supported on transition metal carbides and nitrides. Chem Eng J 426:130781
Pan B, Zhu X, Wu Y et al (2020) Toward highly selective electrochemical CO2 reduction using metal-free heteroatom-doped carbon. Adv Sci 7(16):2001002
Pan F, Yang Y (2020) Designing CO2 reduction electrode materials by morphology and interface engineering. Energy Environ Sci 13(8):2275–2309
Pan F, Zhang H, Liu Z et al (2019) Atomic-level active sites of efficient imidazolate framework-derived nickel catalysts for CO2 reduction. J Mater Chem A 7(46):26231–26237
Pan X, Jiao F, Miao D et al (2021) Oxide–zeolite-based composite catalyst concept that enables syngas chemistry beyond Fischer-Tropsch synthesis. Chem Rev 121(11):6588–6609
Porosoff MD, Chen JG (2013) Trends in the catalytic reduction of CO2 by hydrogen over supported monometallic and bimetallic catalysts. J Catal 301:30–37
Porosoff MD, Yan B, Chen JG (2016) Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: challenges and opportunities. Energy Environ Sci 9(1):62–73
Rosen J, Hutchings GS, Lu Q et al (2015) Mechanistic insights into the electrochemical reduction of CO2 to CO on nanostructured Ag surfaces. ACS Catal 5(7):4293–4299
Shao P, Yi L, Chen S et al (2020) Metal-organic frameworks for electrochemical reduction of carbon dioxide: the role of metal centers. J Energy Chem 40:156–170
Sheng T, Sun S-G (2017) Electrochemical reduction of CO2 into CO on Cu (100): a new insight into the C-O bond breaking mechanism. Chem Commun 53(17):2594–2597
Shi R, Guo J, Zhang X et al (2020) Efficient wettability-controlled electroreduction of CO2 to CO at Au/C interfaces. Nat Commun 11(1):1–10
Wang X, Sun G, Routh P et al (2014) Heteroatom-doped graphene materials: syntheses, properties and applications. Chem Soc Rev 43(20):7067–7098
Yan C, Li H, Ye Y et al (2018) Coordinatively unsaturated nickel–nitrogen sites towards selective and high-rate CO2 electroreduction. Energy Environ Sci 11(5):1204–1210
Yang F, Song P, Liu X et al (2018a) Highly efficient CO2 electroreduction on ZnN4-based single-atom catalyst. Angew Chem Int Ed 57(38):12303–12307
Yang HB, Hung S-F, Liu S et al (2018b) Atomically dispersed Ni (I) as the active site for electrochemical CO2 reduction. Nat Energy 3(2):140–147
Yu W, Porosoff MD, Chen JG (2012) Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts. Chem Rev 112(11):5780–5817
Yu W, Jiang H, Fang J et al (2021a) Designing an electron-deficient Pd/NiCo2O4 bifunctional electrocatalyst with an enhanced hydrodechlorination activity to reduce the consumption of Pd. Environ Sci Technol 55(14):10087–10096
Yu W, Wen L, Gao J et al (2021b) Facile treatment tuning the morphology of Pb with state-of-the-art selectivity in CO2 electroreduction to formate. Chem Commun 57(60):7418–7421
Zhang Q, Kang J, Wang Y (2010) Development of novel catalysts for Fischer-Tropsch synthesis: tuning the product selectivity. ChemCatChem 2(9):1030–1058
Zhang Y, Zeng Z, Li H (2022a) Design of 3d transition metal anchored B5N3 catalysts for electrochemical CO2 reduction to methane. J Mater Chem A 10(17):9737–9745
Zhang Y, Abbas M, Iqbal W (2022b) Perceptions of GHG emissions and renewable energy sources in Europe, Australia and the USA. Environ Sci Pollut Res 29(4):5971–5987
Zhang Y, Yang R, Li H et al (2022c) Boosting electrocatalytic reduction of CO2 to HCOOH on Ni single atom anchored WTe2 monolayer. Small 18(44):2203759
Funding
This work was supported by the National Natural Science Foundation of China (Grants 21908199 and 22076168), the Fundamental Research Funds for the Provincial Universities of Zhejiang (Grant RF-A2020011), and Zhejiang Soft Science Research Program (Grant 2022C15006).
Author information
Authors and Affiliations
Contributions
Weiting Yu: supervision and writing. Jieyun Zhu: data curation and investigation. Sizhuo Chen: formal analysis. Juntao Tang: validation. Jiexu Ye: validation. Shuang Song: supervision and resources.
Corresponding author
Ethics declarations
Ethical approval
Since this study did not recruit any human and/or animal subjects, this section does not apply.
Consent to participate
Since this study did not recruit any human subjects, this section does not apply.
Consent for publication
The authors have not submitted the manuscript to a preprint server before submitting it to Environmental Science and Pollution Research. This work is not under consideration for publication anywhere else and is approved by all co-authors for publication in Environmental Science and Pollution Research.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, W., Zhu, J., Chen, S. et al. Coupling Ni–Cu atomic pair to promote CO2 electroreduction with near-unity CO selectivity. Environ Sci Pollut Res 30, 51876–51886 (2023). https://doi.org/10.1007/s11356-023-25975-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25975-w