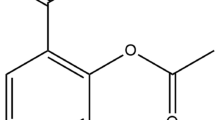
Abstract
This study investigates the feasibility of spent tea waste extract (STWE) as a green modifying agent for the modification of chitosan adsorbent towards aspirin removal. Response surface methodology based on Box-Behnken design was employed to find the optimal synthesis parameters (chitosan dosage, spent tea waste concentration, and impregnation time) for aspirin removal. The results revealed that the optimum conditions for preparing chitotea with 84.65% aspirin removal were 2.89 g of chitosan, 18.95 mg/mL of STWE, and 20.72 h of impregnation time. The surface chemistry and characteristics of chitosan were successfully altered and improved by STWE, as evidenced by FESEM, EDX, BET, and FTIR analysis. The adsorption data were best fitted to pseudo 2nd order, followed by chemisorption mechanisms. The maximum adsorption capacity of chitotea was 157.24 mg/g, as fitted by Langmuir, which is impressive for a green adsorbent with a simple synthesis method. Thermodynamic studies demonstrated the endothermic nature of aspirin adsorption onto chitotea.





















Similar content being viewed by others
Data availability
The datasets and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
AbdMalek NN, Jawad AH, Ismail K, Razuan R, ALOthman ZA (2021) Fly ash modified magnetic chitosan-polyvinyl alcohol blend for reactive orange 16 dye removal: adsorption parametric optimization. IntJ Biol Macromol 189:464–476
Ahmed S, Ikram S (2015) Chitosan & its derivatives: a review in recent innovations. Int J Pharm Sci Res 6:14
Arumugam T, Krishnamoorthy P, Tamilarasan R, Rajagopalan N (2018) Efficient usage of rice husk carbon and chitosan composite for adsorption of rhodamine B from waste water. J Pharm Chem Biol Sci 6:107–121
Atangana E (2019) Adsorption of Zn (II) and Pb (II) ions from aqueous solution using chitosan cross-linked formaldehyde adsorbent to protect the environment. J Polym Environ 27:2281–2291
Aytekin AO, Morimura S, Kida K (2011) Synthesis of chitosan–caffeic acid derivatives and evaluation of their antioxidant activities. J Biosci Bioeng 111:212–216
Banerjee S, Chattopadhyaya MC (2017) Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab J Chem 10:S1629–S1638. https://doi.org/10.1016/j.arabjc.2013.06.005
Bhatia S, Othman Z, Ahmad AL (2007) Pretreatment of palm oil mill effluent (POME) using Moringa oleifera seeds as natural coagulant. J Hazard Mater 145:120–126
Çelebi H, Gök G, Gök O (2020) Adsorption capability of brewed tea waste in waters containing toxic lead (II), cadmium (II), nickel (II), and zinc (II) heavy metal ions. Sci Rep 10:1–12
Chen T, Shi P, Zhang J, Li Y, Duan T, Dai L, Wang L, Yu X, Zhu W (2018) Natural polymer konjac glucomannan mediated assembly of graphene oxide as versatile sponges for water pollution control. Carbohyd Polym 202:425–433
Copello GJ, Mebert AM, Raineri M, Pesenti MP, Diaz LE (2011) Removal of dyes from water using chitosan hydrogel/SiO2 and chitin hydrogel/SiO2 hybrid materials obtained by the sol–gel method. J Hazard Mater 186:932–939
de Sousa DNR, Insa S, Mozeto AA, Petrovic M, Chaves TF, Fadini PS (2018) Equilibrium and kinetic studies of the adsorption of antibiotics from aqueous solutions onto powdered zeolites. Chemosphere 205:137–146
Dehghani MH, Karri RR, Alimohammadi M, Nazmara S, Zarei A, Saeedi Z (2020) Insights into endocrine-disrupting Bisphenol-A adsorption from pharmaceutical effluent by chitosan immobilized nanoscale zero-valent iron nanoparticles. J Mol Liq 311:113317
Delgado N, Capparelli A, Navarro A, Marino D (2019) Pharmaceutical emerging pollutants removal from water using powdered activated carbon: study of kinetics and adsorption equilibrium. J Environ Manage 236:301–308
Dubey SP, Dwivedi AD, Lee C, Kwon Y-N, Sillanpaa M, Ma LQ (2014) Raspberry derived mesoporous carbon-tubules and fixed-bed adsorption of pharmaceutical drugs. J Ind Eng Chem 20:1126–1132
Eidelman RS, Hebert PR, Weisman SM, Hennekens CH (2003) An update on aspirin in the primary prevention of cardiovascular disease. Arch Intern Med 163:2006–2010
Ferrah N, Merghache D, Meftah S, Benbellil S (2022) A new alternative of a green polymeric matrix chitosan/alginate-polyethyleniminemethylene phosphonic acid for pharmaceutical residues adsorption. Environ Sci Pollut Res 29:13675–13687
Gao L, Sun J, Li Y (2011) Functionalized bimodal mesoporous silicas as carriers for controlled aspirin delivery. J Solid State Chem 184:1909–1914
Grumezescu A (2016) Novel approaches of nanotechnology in food. Academic Press
Guaresti O, García-Astrain C, Aguirresarobe R, Eceiza A, Gabilondo N (2018) Synthesis of stimuli–responsive chitosan–based hydrogels by Diels-Alder cross–linkingclick reaction as potential carriers for drug administration. Carbohyd Polym 183:278–286
Habib BA, Sayed S, Elsayed GM (2018) Enhanced transdermal delivery of ondansetron using nanovesicular systems: fabrication, characterization, optimization and ex-vivo permeation study-Box-Cox transformation practical example. Eur J Pharm Sci 115:352–361
Ho Y-S, Chiu W-T, Wang C-C (2005) Regression analysis for the sorption isotherms of basic dyes on sugarcane dust. Biores Technol 96:1285–1291
Hollmann F, Arends IW (2012) Enzyme initiated radical polymerizations. Polymers 4:759–793
Hu Q, Luo Y (2016) Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohyd Polym 151:624–639
Karimidost S, Moniri E, Miralinaghi M (2019) Thermodynamic and kinetic studies sorption of 5-fluorouracil onto single walled carbon nanotubes modified by chitosan. Korean J Chem Eng 36:1115–1123
Kekes T, Tzia C (2020) Adsorption of indigo carmine on functional chitosan and β-cyclodextrin/chitosan beads: Equilibrium, kinetics and mechanism studies. J Environ Manage 262:110372
Khadir A, Mollahosseini A, Tehrani R, Negarestani M (2020) A review on pharmaceutical removal from aquatic media by adsorption: understanding the influential parameters and novel adsorbents. Sustain Green Chem Process Allied Appl 8:207–265
Koutsopoulou E, Koutselas I, Christidis GE, Papagiannopoulos A, Marantos I (2020) Effect of layer charge and charge distribution on the formation of chitosan-smectite bionanocomposites. Appl Clay Sci 190:105583
Kyzas GZ, Kostoglou M, Lazaridis NK, Lambropoulou DA, Bikiaris DN (2013) Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem Eng J 222:248–258
Kyzas GZ, Bikiaris DN (2015) Recent modifications of chitosan for adsorption applications: a critical and systematic review. Mar Drugs 13:312–337
Laysandra L et al (2019) Highly adsorptive chitosan/saponin-bentonite composite film for removal of methyl orange and Cr (VI). Environ Sci Pollut Res 26:5020–5037
Lessa EF, Nunes ML, Fajardo AR (2018) Chitosan/waste coffee-grounds composite: an efficient and eco-friendly adsorbent for removal of pharmaceutical contaminants from water. Carbohyd Polym 189:257–266
Li C, Cui J, Wang F, Peng W, He Y (2016) Adsorption removal of Congo red by epichlorohydrin-modified cross-linked chitosan adsorbent. Desalin Water Treat 57:14060–14066
Li Y, Li L, Chen T, Duan T, Yao W, Zheng K, Dai L, Zhu W (2018) Bioassembly of fungal hypha/graphene oxide aerogel as high performance adsorbents for U (VI) removal. Chem Eng J 347:407–414
Liakos EV, Lazaridou M, Michailidou G, Koumentakou I, Lambropoulou DA, Bikiaris DN, Kyzas GZ (2021) Chitosan adsorbent derivatives for pharmaceuticals removal from effluents: a review. Macromol 1:130–154
Liang J, Li F, Fang Y, Yang W, An X, Zhao L, Xin Z, Cao L, Hu Q (2011) Synthesis, characterization and cytotoxicity studies of chitosan-coated tea polyphenols nanoparticles. Colloids Surf B 82:297–301
Lipton RB, Stewart WF, Ryan RE, Saper J, Silberstein S, Sheftell F (1998) Efficacy and safety of acetaminophen, aspirin, and caffeine in alleviating migraine headache pain: three double-blind, randomized, placebo-controlled trials. Arch Neurol 55:210–217
Liu S, Wang Z, Song P (2018) Free radical graft copolymerization strategy to prepare catechin-modified chitosan loose nanofiltration (NF) membrane for dye desalination. ACS Sustain Chem Eng 6:4253–4263
Lu S-Y, Qian J-Q, Wu Z-G, Ye W-D, Wu G-F, Pan Y-B, Zhang K-Y (2009) Application of statistical method to evaluate immobilization variables of trypsin entrapped with sol-gel method. J Biochem Technol 1:79–84
Ma J, Xin C, Tan C (2015) Preparation, physicochemical and pharmaceutical characterization of chitosan from Catharsius molossus residue. Int J Biol Macromol 80:547–556
Maran JP (2015) Statistical optimization of aqueous extraction of pectin from waste durian rinds. Int J Biol Macromol 73:92–98
Masjoudi M, Golgoli M, Nejad ZG, Sadeghzadeh S, Borghei SM (2021) Pharmaceuticals removal by immobilized laccase on polyvinylidene fluoride nanocomposite with multi-walled carbon nanotubes. Chemosphere 263:128043
Mishra AK (2016) Smart materials for waste water applications. John Wiley & Sons
Mochizuki M, Yamazaki S-i, Kano K, Ikeda T (2002) Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochimica et Biophysica Acta (BBA)-General Subjects 1569:35–44
Moghiseh Z, Rezaee A, Ghanati F, Esrafili A (2019) Metabolic activity and pathway study of aspirin biodegradation using a microbial electrochemical system supplied by an alternating current. Chemosphere 232:35–44
Mojiri A, Andasht Kazeroon R, Gholami A (2019) Cross-linked magnetic chitosan/activated biochar for removal of emerging micropollutants from water: optimization by the artificial neural network. Water 11:551
Mompelat S, Le Bot B, Thomas O (2009) Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ Int 35:803–814
Mucha M, Mucha M (2017) Ibuprofen and acetylsalicylic acid biosorption on the leaves of the knotweed Fallopia x bohemica. New J Chem 41:7953–7959
Nagaonkar D, Gaikwad S, Rai M (2015) Catharanthus roseus leaf extract-synthesized chitosan nanoparticles for controlled in vitro release of chloramphenicol and ketoconazole. Colloid Polym Sci 293:1465–1473
Nordin AH, Ngadi N, Othman NFH, Razali NA, Nabgan W, Alam MNHZ, Wong S, An EH. (2020a) Adsorptive Removal of Acetylsalicylic Acid in Wastewater Onto Crosslinked-Chitosan. Journal of Advances in Engineering Research 200:132–137
Nordin AH, Wong S, Ngadi N, Zainol MM, Abd Latif NAF, Nabgan W (2020b) Surface functionalization of cellulose with polyethyleneimine and magnetic nanoparticles for efficient removal of anionic dye in wastewater. J Environ Chem Eng 9:104639
Omidinasab M, Rahbar N, Ahmadi M, Kakavandi B, Ghanbari F, Kyzas GZ, Martinez SS, Jaafarzadeh N (2018) Removal of vanadium and palladium ions by adsorption onto magnetic chitosan nanoparticles. Environ Sci Pollut Res 25:34262–34276
Ostovan A, Ghaedi M, Arabi M (2018) Fabrication of water-compatible superparamagnetic molecularly imprinted biopolymer for clean separation of baclofen from bio-fluid samples: a mild and green approach. Talanta 179:760–768
Pasanphan W, Chirachanchai S (2008) Conjugation of gallic acid onto chitosan: an approach for green and water-based antioxidant. Carbohyd Polym 72:169–177
Quesada HB, Baptista ATA, Cusioli LF, Seibert D, de Oliveira BC, Bergamasco R (2019) Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: a review. Chemosphere 222:766–780
Quideau S, Deffieux D, Douat-Casassus C, Pouységu L (2011) Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed 50:586–621
Quignard F, Valentin R, Di Renzo F (2008) Aerogel materials from marine polysaccharides. New J Chem 32:1300–1310
Rao P, Knaus EE (2008) Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci 11:81s–110s
Razmi FA, Ngadi N, Wong S, Inuwa IM, Opotu LA (2019) Kinetics, thermodynamics, isotherm and regeneration analysis of chitosan modified pandan adsorbent. J Clean Prod 231:98–109
Riegger BR, Bäurer B, Mirzayeva A, Tovar GE, Bach M (2018) A systematic approach of chitosan nanoparticle preparation via emulsion crosslinking as potential adsorbent in wastewater treatment. Carbohyd Polym 180:46–54
Ritu N, Asheesh S, Dinesh B (2012) Aspirin: an overview of randomized controlled trials. Int J Res Pharm Sci 2:53–67
Saheed IO, Da Oh W, Suah FBM (2021) Chitosan modifications for adsorption of pollutants–a review. J Hazard Mater 408:124889
Saleh A, Mukhtar SA, Fawwaz M, Pratama M, Kosman R, Naid T (2015) Deacetylation degree of chitosan by various bases and its metal adsorption ability related on antioxidant activity. J Chem Pharm Res 7:265–269
Satoh E, Tohyama N, Nishimura M (2005) Comparison of the antioxidant activity of roasted tea with green, oolong, and black teas. Int J Food Sci Nutr 56:551–559
Shukla SK, Pandey S, Saha S, Singh HR, Mishra PK, Kumar S, Jha SK (2021) Removal of crystal violet by Cu-chitosan nano-biocomposite particles using Box-Behnken design. J Environ Chem Eng 9:105847
Sohilait HJ, Kainama H (2015) Synthesis of myristicin ketone (3, 4-methylenedioxy-5-methoxyphenyl)-2-propanone from myristicin. Science 3:62–66
Tahira I, Aslam Z, Abbas A, Monim-ul-Mehboob M, Ali S, Asghar A (2019) Adsorptive removal of acidic dye onto grafted chitosan: a plausible grafting and adsorption mechanism. Int J Biol Macromol136:1209–1218
Tang D-W, Yu S-H, Ho Y-C, Huang B-Q, Tsai G-J, Hsieh H-Y, Sung H-W, Mi F-L (2013) Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocolloids 30:33–41
Tzereme A, Christodoulou E, Kyzas GZ, Kostoglou M, Bikiaris DN, Lambropoulou DA (2019) Chitosan grafted adsorbents for diclofenac pharmaceutical compound removal from single-component aqueous solutions and mixtures. Polymers 11:497
Vakili M, Rafatullah M, Salamatinia B, Abdullah AZ, Ibrahim MH, Tan KB, Gholami Z, Amouzgar P (2014) Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohyd Polym 113:115–130
Verma M, Lee I, Kumar V, Pan S-Y, Fan C, Kim H (2022) Chitosan cross-linked β–cyclodextrin polymeric adsorbent for the removal of perfluorobutanesulfonate from aqueous solution: adsorption kinetics, isotherm, and mechanism. Environ Sci Pollut Res 30:1–10
Wang F, Gao J, Jia L, Wang S, Ning P (2022) Green synthesis of a novel functionalized chitosan adsorbent for Cu (II) adsorption from aqueous solution. Environ Sci Pollut Res 29:989–998
Wang J, Chen C (2014) Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides. Biores Technol 160:129–141
Wei Z, Zhang Y, Ma X, Wang W (2021) Insight into the high-efficiency adsorption of pyrene by Schiff base porous polymers: Modelling and mechanism. Polymer 220:123576
Wong S, Lee Y, Ngadi N, Inuwa IM, Mohamed NB (2018) Synthesis of activated carbon from spent tea leaves for aspirin removal. Chin J Chem Eng 26:1003–1011
Xiao C, Li H, Zhao Y, Zhang X, Wang X (2020) Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes. J Environ Manage 275:111262
Xie W, Xu P, Liu Q (2001) Antioxidant activity of water-soluble chitosan derivatives. Bioorg Med Chem Lett 11:1699–1701
Zhang D, Crini G, Lichtfouse E, Rhimi B, Wang C (2020a) Removal of mercury ions from aqueous solutions by crosslinked chitosan-based adsorbents: a mini review. Chem Rec 20:1220–1234
Zhang F, Wang B, Jie P, Zhu J, Cheng F (2021) Preparation of chitosan/lignosulfonate for effectively removing Pb (II) in water. Polymer 228:123878
Zhang R, Li Y, Wang Z, Tong Y, Sun P (2020b) Biochar-activated peroxydisulfate as an effective process to eliminate pharmaceutical and metabolite in hydrolyzed urine. Water Res 177:115809
Zhu B, Li J, He Y, Yoshie N, Inoue Y (2003) Hydrogen-bonding interaction and crystalline morphology in the binary blends of poly (ε-caprolactone) and polyphenol catechin. Macromol Biosci 3:684–693
Zhu W, Zhang Z (2014) Preparation and characterization of catechin-grafted chitosan with antioxidant and antidiabetic potential. Int J Biol Macromol 70:150–155
Zhuang S, Zhu K, Wang J (2021) Fibrous chitosan/cellulose composite as an efficient adsorbent for Co (II) removal. J Clean Prod 285:124911
Acknowledgements
The first author, Abu Hassan Nordin, wishes to express his heartfelt gratitude to Universiti Teknologi Malaysia for the financial assistance provided under the Zamalah scholarship scheme.
Funding
This work was funded by a Research University grant from Universiti Teknologi Malaysia (Vot no. Q.J130000.3851.18J95).
Author information
Authors and Affiliations
Contributions
Abu Hassan Nordin: data curation, investigation, formal analysis, and writing—original draft; Norzita Ngadi: supervision, funding acquisition and resources; Ahmad Ilyas Rushdan: supervision, writing—review and editing; Nur Aien Fatini Abd Latif: data curation; Muhammad Luqman Nordin: conceptualization; Mohd Syahlan Mohd Syukri: writing—review and editing, validation; Walid Nabgan: visualization; Syafikah Huda Paiman: writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nordin, A.H., Ngadi, N., Ilyas, R.A. et al. Green surface functionalization of chitosan with spent tea waste extract for the development of an efficient adsorbent for aspirin removal. Environ Sci Pollut Res 30, 125048–125065 (2023). https://doi.org/10.1007/s11356-023-25816-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25816-w