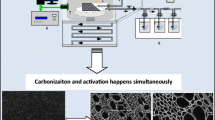
Abstract
Microwave co-pyrolysis of sewage sludge and leucaena wood was conducted to produce biochar as an adsorbent for CO2 capture. Both microwave power level and blending ratio were crucial factors affecting the CO2 adsorption capacity of biochar. At a power level of 150 W, the biochar produced by microwave co-pyrolysis of 25% sewage sludge and 75% leucaena wood possessed the highest CO2 adsorption capacity. When the biochar was produced at 100 W, its CO2 adsorption capacity was higher than predicted. Based on the proximate and elemental compositions of biochar, two equations were obtained to predict CO2 adsorption capacity. The proximate composition of biochar can provide more precise prediction of CO2 adsorption capacity than elemental composition according to the higher R2 value provided. The blending ratio of 50% would be most appropriate to produce the biochar with acceptable reduction in CO2 adsorption capacity and loss of quantity. The pseudo-second-order model would be most suitable for simulating the kinetic of CO2 adsorption. The biochar produced from 1 metric tonne of sewage sludge and leucaena wood can offset carbon tax by 83 US dollars. Based on experimental results and findings, microwave co-pyrolysis should be a feasible technique to produce biochar possessing high CO2 adsorption capacity.





Similar content being viewed by others
Data availability
Not applicable.
References
Appleton TJ, Colder RI, Kingman SW, Lowndes IS, Read AG (2005) Microwave technology for energy-efficient processing of waste. Appl Energy 81:85–113
Barneto AG, Carmona JA, Alfonso JEM, Alcaide LJ (2009) Use of autocatalytic kinetics to obtain composition of lignocellulosic materials. Bioresour Technol 100:3963–3973
Cha JS, Park SH, Jung SC, Ryu C, Jeon JK, Shin MC, Park YK (2016) Production and utilization of biochar: a review. J Ind Eng Chem 40:1–15
Chien SH, Clayton WR (1980) Application of Elovich equation to the kinetics of phosphate release and sorption on soils. Soil Sci Soc Am J 44:265–268
Cornelissen G, Rutherford DW, Arp HPH, Dorsch P, Kelly CN, Rostad CE (2013) Sorption of pure N2O to biochars and other organic and inorganic materials under anhydrous conditions. Environ Sci Technol 47:7704–7712
Creamer AE, Gao B, Zhang M (2014) Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem Eng J 249:174–179
Creamer AE, Gao B, Wang S (2016) Carbon dioxide capture using various metal oxyhydroxide–biochar composites. Chem Eng J 283:826–832
Dominguez A, Menendez JA, Inguanzo M, Bernad PL, Pis JJ (2003) Gas chromatographic–mass spectrometric study of the oil fractions produced by microwave-assisted pyrolysis of different sewage sludges. J Chromatogr A 1012:193–206
Ghiat I, Al-Ansari T (2021) A review of carbon capture and utilisation as a CO2 abatement opportunity within the EWF nexus. J CO2 Util 45:101432.
Guo L, Yang J, Hu G, Hu X, DaCosta H, Fan M (2016) CO2 removal from flue gas with amine-impregnated titanate nanotubes. Nano Energy 25:1–8
Haque KE (1999) Microwave energy for mineral treatment processes—a brief review. Int J Miner Process 57:1–24
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Ho YS, Ng JCY, McKay G (2000) Kinetics of pollutant sorption by biosorbents: review. Sep Purif Rev 29:189–232
Huang YF, Chiueh PT, Kuan WH, Lo SL (2015a) Effects of lignocellulosic composition and microwave power level on the gaseous product of microwave pyrolysis. Energy 89:974–981
Huang YF, Chiueh PT, Shih CH, Lo SL, Sun L, Zhong Y, Qiu C (2015b) Microwave pyrolysis of rice straw to produce biochar as an adsorbent for CO2 capture. Energy 84:75–82
Huang YF, Sung HT, Chiueh PT, Lo SL (2016) Co-torrefaction of sewage sludge and leucaena by using microwave heating. Energy 116:1–7
IPCC (2007) Climate change 2007: impacts, adaptation and vulnerability. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, editors. Cambridge University Press, Cambridge, UK.
Jhong HRM, Ma S, Kenis PJA (2013) Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Curr Opin Chem Eng 2:191–199
Jones DA, Lelyveld TP, Mavrofidis SD, Kingman SW, Miles NJ (2002) Microwave heating applications in environmental engineering—a review. Resour Conserv Recycl 34:75–90
Lam SS, Chase HA (2012) A review on waste to energy processes using microwave pyrolysis. Energies 5:4209–4232
Liu Q, Shi J, Zheng S, Tao M, He Y, Shi Y (2014) Kinetics studies of CO2 adsorption/desorption on amine-functionalized multiwalled carbon nanotubes. Ind Eng Chem Res 53:11677–11683
Liu N, Charrua AB, Weng CH, Yuan X, Ding F (2015) Characterization of biochars derived from agricultural wastes and their adsorptive removal of atrazine from aqueous solution: a comparative study. Bioresour Technol 198:55–62
Low MJD (1960) Kinetics of chemisorption of gases on solids. Chem Rev 60:267–312
Mushtaq F, Mat R, Ani FN (2014) A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renew Sustain Energy Rev 39:555–574
Plaza MG, Gonzalez AS, Pevida C, Pis JJ, Rubiera F (2012) Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Appl Energy 99:272–279
Rashidi NA, Yusup S, Hameed BH (2013a) Kinetic studies on carbon dioxide capture using lignocellulosic based activated carbon. Energy 61:440–446
Rashidi NA, Yusup S, Lam HL (2013b) Kinetic studies on carbon dioxide capture using activated carbon. Chem Eng Trans 35:361–366
Samanta A, Zhao A, Shimizu GKH, Sarkar P, Gupta R (2012) Post-combustion CO2 capture using solid sorbents: a review. Ind Eng Chem Res 51:1438–1463
Serafin J, Narkiewicz U, Morawski AW, Wróbel RJ, Michalkiewicz B (2017) Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J CO2 Util 18:73–79.
Tewari SK, Katiyar RS, Ram B, Misra PN (2004) Effect of age and season of harvesting on the growth, coppicing characteristics and biomass productivity of Leucaena leucocephala and Vitex negundo. Biomass Bioenerg 26:229–234
Vijayakumar G, Tamilarasan R, Dharmendirakumar M (2012) Adsorption, kinetic, equilibrium and thermodynamic studies on the removal of basic dye Rhodamine-B from aqueous solution by the use of natural adsorbent perlite. J Mater Environ Sci 3:157–170
Wang L, Rao L, Xia B, Wang L, Yue L, Liang Y, DaCosta H, Hu X (2018) Highly efficient CO2 adsorption by nitrogen-doped porous carbons synthesized with low-temperature sodium amide activation. Carbon 130:31–40
Weber WJ Jr, Morriss JC (1963) Kinetics of adsorption on carbon from solution. J Sanitary Eng Div Am Soc Civil Eng 89:31–60
Werther J, Ogada T (1999) Sewage sludge combustion. Prog Energy Combust Sci 25:55–116
World Bank (2020) State and trends of carbon pricing 2020. World Bank, Washington, DC
Yin C (2012) Microwave-assisted pyrolysis of biomass for liquid biofuels production. Bioresour Technol 120:273–284
Zhang J, Yin R, Shao Q, Zhu T, Huang X (2019) Oxygen vacancies in amorphous InOx nanoribbons enhance CO2 adsorption and activation for CO2 electroreduction. Angew Chem Int Ed 58:1–6
Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan, ROC [grant number 111-2221-E-002-038].
Funding
This work was supported by the Ministry of Science and Technology, Taiwan, ROC [grant number 111–2221-E-002–038].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yu-Fong Huang and Pei-Te Chiueh. The first draft of the manuscript was written by Yu-Fong Huang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors agree to publish this work in ESPR.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Zhihong Xu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, YF., Chiueh, PT. & Lo, SL. Carbon capture of biochar produced by microwave co-pyrolysis: adsorption capacity, kinetics, and benefits. Environ Sci Pollut Res 30, 22211–22221 (2023). https://doi.org/10.1007/s11356-022-23734-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23734-x