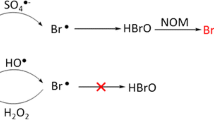
Abstract
Bromate (BrO3−) and ammonia nitrogen (NH4+) are both typical environmental pollutants: BrO3− has been categorized as one of the Group 2B carcinogen by IARC; an excess of NH4+ might result in the eutrophication of water. The existence of NH4+ could inhibit the transformation of bromide (Br−) to bromate (BrO3−). However, the interaction of NH4+ and BrO3− during the removal process is not clear. This study intends to disclose the mutual relationships of ammonia nitrogen and bromate ions under UV irradiation or UV/TiO2 conditions. Without UV irradiation, BrO3− and NH4+ were both stable even under the presentation of each other. Under UV irradiation or UV/TiO2 conditions, BrO3− and NH4+ promoted the degradation of each other, showing the synergistic degradation mechanism. In the neutral environment, both of BrO3− and NH4+ could be transformed effectively. Furthermore, NH4+ accelerated the transformation of BrO3− to Br− at the reaction beginning and the existence of BrO3− is beneficial for the transformation of NH4+ to N2.









Similar content being viewed by others
Data availability
The data used to support the outcomes of this study are included within the article.
References
Antoniou MG, Sichel C, Andre K, Andersen HR (2016) Novel pre-treatments to control bromate formation during ozonation. J Hazard Mater 323:452–459
Ateia M, Erdem CU, Ersan MS, Ceccato M, Karanfil T (2019) Selective removal of bromide and iodide from natural waters using a novel AgCl-SPAC composite at environmentally relevant conditions. Water Res 156:168–178
Camera-Roda G, Loddo V, Palmisano L, Parrino F (2019) Photocatalytic ozonation for a sustainable aquaculture: a long-term test in a seawater aquarium. Appl Catal B-Environ 253:69–76
Chen X, Yang H, Au C, Tian S, Xiong Y, Chang Y (2020) Efficiency and mechanism of pollutant degradation and bromate inhibition by faceted CeO2 catalyzed ozonation: experimental and theoretical study. Chem Eng J 390:124480
Cui M, Choi J, Lee Y, Ma J, Kim D, Choi J, Jang M, Khim J (2017) Significant enhancement of bromate removal in drinking water: implications for the mechanism of sonocatalytic reduction. Chem Eng J 317:404–412
da Silva VM, da Cunha Veloso MC, de Oliveira AS, Santos GVP, de Pereira PAP, de Andrade JB (2005) Determination of simple bromophenols in marine fishes by reverse-phase high performance liquid chromatography (RP-HPLC). Talanta 68(2):323–328
Di G, Zhu Z, Zhang H, Qiu Y, Yin D, Crittenden J (2020) Simultaneous sulfamethazine oxidation and bromate reduction by Pd- mediated Z-scheme Bi2MoO6/g-C3N4 photocatalysts: Synergetic mechanism and degradative pathway. Chem Eng J 401:126061
Du T, Zhang G, Zou J (2022) Coupling photocatalytic and electrocatalytic oxidation towards simultaneous removal of humic acid and ammonia-nitrogen in landscape water. Chemosphere 286:131717
Eusebi AL, Battistoni P (2016) The ozone treatment for nitrogen removal from liquid wastes at high salinity: full-scale optimization and economical aspect. Ozone: Sci Eng 38(3):219–224
Fakioglu M, Gulhan H, Ozgun H, Ersahin ME, Ozturk I (2020) Determination of optimum operational conditions for the removal of 2-MIB from drinking water by peroxone process: a pilot-scale study. Water Supply 20(6):2339–2347
Flury M, Papritz A (1993) Bromide in the natural environment: occurrence and toxicity. J Environ Qual 22(4):747–758
Hu Y, Wang M, Hu F, Wu J, Xu L, Xu G, Jian Y, Peng X (2020) Controllable construction of hierarchical TiO2 supported on hollow rGO/P-HC heterostructure for highly efficient photocatalysis. Colloids Surf A-Physicochem Eng Asp 598:124831
Hua L, Yuhang Y, Yamei H, Minmin J, Kun D (2021) The influence factors of simultaneous removal of bromate and perchlorate by hydrogen-based membrane biofilm reactor. Technol Water Treat 47(02):112–118
Huang L, Li L, Dong W, Liu Y, Hou H (2008) Removal of ammonia by OH radical in aqueous phase. Environ Sci Technol 42(21):8070–8075
Huang X, Wang L, Zhou J, Gao N (2014) Photocatalytic decomposition of bromate ion by the UV/P25-graphene processes. Water Res 57:1–7
Huang H, Liu G, Wang X (2020) A novel zero valent metal bismuth for bromate removal: direct and ultraviolet enhanced reduction. RSC Adv 10(7):4148–4155
IARC (1999) Potassium bromate. IARC Monogr Eval Carcinog Risks Hum 73:481–496
Jahan BN, Li L, Pagilla KR (2021) Fate and reduction of bromate formed in advanced water treatment ozonation systems: a critical review. Chemosphere 266:128964
Jung B, Nicola R, Batchelor B, Abdel-Wahab A (2014) Effect of low- and medium-pressure Hg UV irradiation on bromate removal in advanced reduction process. Chemosphere 117:663–672
Ke J, Liu J, Sun H, Zhang H, Duan X, Liang P, Li X, Tade MO, Liu S, Wang S (2017) Facile assembly of Bi2O3/Bi2S3/MoS2 n-p heterojunction with layered n-Bi2O3 and p-MoS2 for enhanced photocatalytic water oxidation and pollutant degradation. Appl Catal B-Environ 200:47–55
Kumar A, Sharma G, Naushad M, Ahamad T, Veses RC, Stadler FJ (2019) Highly visible active Ag2CrO4/Ag/BiFeO3@RGO nano-junction for photoreduction of CO2 and photocatalytic removal of ciprofloxacin and bromate ions: the triggering effect of Ag and RGO. Chem Eng J 370:148–165
Lakhian V, Dickson-Anderson SE (2020) Reduction of bromate and chlorate contaminants in water using aqueous phase corona discharge. Chemosphere 255:126864
Lem O, Yoon S, Bae S, Lee W (2021) The enhanced reduction of bromate by highly reactive and dispersive green nano-zerovalent iron (G-NZVI) synthesized with onion peel extract. RSC Adv 11(9):5008–5018
Lin D, Liang H, Li G (2020) Factors affecting the removal of bromate and bromide in water by nanofiltration. Environ Sci Pollut Res 27(20):24639–24649
Ling L, Li Z, Fang J, Shang C (2018) Controlling bromate formation in the Co(II)/peroxymonosulfate process by ammonia, chlorine-ammonia and ammonia-chlorine pretreatment strategies. Water Res 139:220–227
Liu G, You S, Zhang Y, Huang H, Spanjers H (2019) Conjugated donor-acceptor (D-A) supramolecule catalyst for visible light-driven photocatalytic removal of bromate in water. J Colloid Interface Sci 553:666–673
Liu G, Zhu Y, Yan Q, Wang H, Wu P, Shen Y, Doekhi-Bennani Y (2021) Tuning electron transfer by crystal facet engineering of BiVO4 for boosting visible-light driven photocatalytic reduction of bromate. Sci Total Environ 762:143086
Luo X, Chen C, Jing Y, Wang J, Wang C (2015) Characterization of La/Fe/TiO2 and its photocatalytic performance in ammonia nitrogen wastewater. Int J Environ Res Public Health 12(11):14626–14639
Peng X, Wang M, Dai H, Qiu F, Hu F (2020) In situ growth of carbon nitride on titanium dioxide/hemp stem biochar toward 2D heterostructured photocatalysts for highly photocatalytic activity. Environ Sci Pollut Res 27(31):39198–39210
Pretzer LA, Carlson PJ, Boyd JE (2008) The effect of Pt oxidation state and concentration on the photocatalytic removal of aqueous ammonia with Pt-modified titania. J Photochem Photobiol, A 200(2–3):246–253
Shanmugavel V, Komala Santhi K, Kurup AH, Kalakandan S, Anandharaj A, Rawson A (2020) Potassium bromate: effects on bread components, health, environment and method of analysis: A review. Food Chem 311:125964
Siddiqui MS, Amy GL, McCollum LJ (1996) Bromate destruction by uv irradiation and electric arc discharge. Ozone: Sci Eng 18(3):271–290
Soltermann F, Abegglen C, Tschui M, Stahel S, von Gunten U (2017) Options and limitations for bromate control during ozonation of wastewater. Water Res 116:76–85
Suttiponparnit K, Jiang J, Sahu M, Suvachittanont S, Charinpanitkul T, Biswas P (2011) Role of surface area, primary particle size, and crystal phase on Titanium Dioxide nanoparticle dispersion properties. Nanaoscale Res Lett 6:27
Tanaka J, Matsumura M (2003) Application of ozone treatment for ammonia removal in spent brine. Adv Environ Res 7(4):835–845
Von Gunten U, Oliveras Y (1998) Advanced oxidation of bromide-containing waters: bromate formation mechanisms. Environ Sci Technol 32(1):63–70
Walpen N, Joss A, von Gunten U (2022) Application of UV absorbance and electron-donating capacity as surrogates for micropollutant abatement during full-scale ozonation of secondary-treated wastewater. Water Res 209:117858
Wang CY, Zeng T, Zhu SP, Gu CT (2019) Synergistic mechanism of rare-earth modification TiO2 and photodegradation on benzohydroxamic acid. Appl Sci 9(2):339
Wen G, Wang S, Wang T, Feng Y, Chen Z, Lin W, Huang T, Ma J (2020) Inhibition of bromate formation in the O3/PMS process by adding low dosage of carbon materials: Efficiency and mechanism. Chem Eng J 402:126207
Williams MD, Coffey BM, Krasner SW (2003) Evaluation of pH and ammonia for controlling bromate during “Cryptosporidium” disinfection. Journal (american Water Works Association) 95(10):82–93
Wu Q-Y, Yang L-L, Zhang X-Y, Wang W-L, Lu Y, Du Y, Lu Y, Hu H-Y (2020) Ammonia-mediated bromate inhibition during ozonation promotes the toxicity due to organic byproduct transformation. Environ Sci Technol 54(14):8926–8937
Xiao J, Yang W, Li Q (2017a) Bi quantum dots on rutile TiO2 as hole trapping centers for efficient photocatalytic bromate reduction under visible light illumination. Appl Catal B-Environ 218:111–118
Xiao Q, Wang T, Yu S, Yi P, Li L (2017b) Influence of UV lamp, sulfur(IV) concentration, and pH on bromate degradation in UV/sulfite systems: mechanisms and applications. Water Res 111:288–296
Xiao Q, Yu S, Li L, Zhang Y, Yi P (2019) Degradation of bromate by Fe(II)-Ti(IV) layered double hydroxides nanoparticles under ultraviolet light. Water Res 150:310–320
Xu Y, He Z, Yu S, Li L, Cai L, Yi P (2022) Advanced reduction of bromate by UV/TiO2-Bi process without external sacrificial agents: mechanism and applications. Chem Eng J 429:132104
Yang J, Li J, Dong W, Ma J, Yang Y, Li J, Yang Z, Zhang X, Gu J, Xie W, Cang Y (2017) Enhancement of bromate formation by pH depression during ozonation of bromide-containing water in the presence of hydroxylamine. Water Res 109:135–143
Yang J, Dong Z, Jiang C, Wang C, Liu H (2019) An overview of bromate formation in chemical oxidation processes: occurrence, mechanism, influencing factors, risk assessment, and control strategies. Chemosphere 237:124521
Yao F, Yang Q, Sun J, Chen F, Zhong Y, Yin H, He L, Tao Z, Pi Z, Wang D, Li X (2020) Electrochemical reduction of bromate using noble metal-free nanoscale zero-valent iron immobilized activated carbon fiber electrode. Chem Eng J 389:123588
Yu J, Wang Y, Wang Q, Wang Z, Zhang D, Yang M (2020) Implications of bromate depression from H2O2 addition during ozonation of different bromide-bearing source waters. Chemosphere 252:126596
Zhang X, Zhang T, Ng J, Pan JH, Sun DD (2010) Transformation of bromine species in TiO2 photocatalytic system. Environ Sci Technol 44(1):439–444
Zhang Y, Li L, Liu H, Lu T (2017) Graphene oxide and F co-doped TiO2 with (001) facets for the photocatalytic reduction of bromate: synthesis, characterization and reactivity. Chem Eng J 307:860–867
Zhang Y, Xia Y, Li Q, Qi F, Xu B, Chen Z (2018) Synchronously degradation benzotriazole and elimination bromate by perovskite oxides catalytic ozonation: performance and reaction mechanism. Sep Purif Technol 197:261–270
Zhang F, Zhang D, Chang T, Li H, Cui J, Cui J (2020a) Bromate formation by the oxidation of bromide in the electrochemically activated persulfate process: mechanism and influencing factors. Int J Electrochem Sci 15(8):7282–7297
Zhang Y, Li J, Liu H (2020b) Synergistic removal of bromate and ibuprofen by graphene oxide and TiO2 heterostructure doped with F: performance and mechanism. J Nanomater 2020:6094984
Funding
This research was financially supported by the National Key Research and Development Program of China (2019YFC1805100), the Innovation and Entrepreneurship Training Program for College Students of Jiangxi Province (S202010407002), and the Program of Qingjiang Excellent Young Talents, JXUST.
Author information
Authors and Affiliations
Contributions
Yiting Zeng and Jin Zeng repeated the experiments. Zhenwei Luo made a great contribution to the writing of the manuscript. Jun Liu analyzed the experiment data. Chunying Wang was the major writer of the manuscript. The elementary experiments were operated by Jiahao Pan and Yuxia Luo. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors equally participate in the study.
Consent for publication
All authors allow the publication of the paper.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, Y., Zeng, J., Luo, Z. et al. Degradation mechanism of ammonia nitrogen synergistic with bromate under UV or UV/TiO2. Environ Sci Pollut Res 30, 22284–22295 (2023). https://doi.org/10.1007/s11356-022-23658-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23658-6