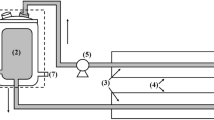
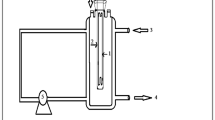
Abstract
Hydrochlorothiazide (HCT) is a pharmaceutical micropollutant highly toxic to the environment, being absolutely necessary to oxidize it completely to CO2. Here, the variables stoichiometric H2O2 excess for (a) degradation and (b) mineralization are defined and used as metric to quantify the dosimetry of the H2O2. So that, dose of H2O2 qualifies being under- and over-dose respectively for values below and above such standards. In this work, these concepts have been elucidated across AOPs regarding the H2O2 degradation excess, whereas only UVC-Fenton was used regarding the H2O2 mineralization excess. At a H2O2 mineralization excess of 0.68 (equivalent to degradation excess of 36.74), oxidation via UVC-H2O2 enables absolute (100%) HCT degradation within 60 min; however, the mineralization of HCT demonstrated limited optimization for all AOPs employed in the beaker-like reactor of this work, being the underlying reasons investigated hereby. At best, 26.70% HCT mineralization was observed within 60 min of UVC photo-Fenton using an initial 2.00 H2O2 mineralization excess. Such mineralization of 26.7% is unexpectedly low considering that, in addition, the residual H2O2 concentration almost fully depletes within 30 min of UVC-Fenton oxidation. Taken all that together, the loss of H2O2 due its decomposition induced by the risen temperature from 28 to 70ºC very likely were the underlying reason preventing better mineralization performance. We successfully demonstrated 18% of mean efficiency of radical •OH consumption signals that the overheating is indeed a designer problem with the photo-reactor since a well-refrigerated photo-reactor shows a mean efficiency of 38% for the same H2O2 excess.
Graphical abstract








Similar content being viewed by others
Data availability
Not applicable.
References
Biel-Maeso M et al (2018) Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain). Sci Total Environ 612:649–659
Borowska E, Bourgin M, Hollender J, Kienle C, McArdell CS, von Gunten U (2016) Oxidation of cetirizine, fexofenadine and hydrochlorothiazide during ozonation: Kinetics and formation of transformation products. Water Res 94:350–362
Brillas E, Garcia-Segura S (2020) Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: a review on the relevance of phenol as model molecule. Sep Purif Technol 237:116337
Brillas E, Sirés I, Oturan MA (2009) Electro-fenton process and related electrochemical technologies based on fenton’s reaction chemistry. Chem Rev 109(12):6570–6631
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. J Phys Chem Ref Data 17(2):513–886
Castiglioni S et al (2005) A multiresidue analytical method using solid-phase extraction and high-pressure liquid chromatography tandem mass spectrometry to measure pharmaceuticals of different therapeutic classes in urban wastewaters. J Chromatography A 1092(2):206–215
Contreras N, Vidal J, Berríos C, Villegas L, Salazar R (2015) Degradation of antihypertensive hydrochlorothiazide in water from pharmaceutical formulations by electro-oxidation using a BDD anode. Int J Electrochem Sci 10:9269–9285
Cunha-Filho FJV, Mota-Lima A, Ratkievicius LA, Silva DJ, Silva DN, Chiavone-Filho O, Oller do Nascimento CA (2019) Rapid mineralization rate of acetylsalicylic acid in a tubular photochemical reactor: the role of the optimized excess of H2O2. J Water Process Eng 31:100856
da Silva SS, Chiavone-Filho O, de Barros Neto EL, Mota ALN, Foletto EL, Nascimento CAO (2014) Photodegradation of non-ionic surfactant with different ethoxy groups in aqueous effluents by the photo-Fenton process. Environ Technol 35(12):1556–1564
Duan X, Yang S, Wacławek S, Fang G, Xiao R, Dionysiou DD (2020) Limitations and prospects of sulfate-radical based advanced oxidation processes. J Environ Chem Eng 8(4):103849
Fernández-Perales M, Sánchez-Polo M, Rozalen M, López-Ramón MV, Mota AJ, Rivera-Utrilla J (2020) Degradation of the diuretic hydrochlorothiazide by UV/Solar radiation assisted oxidation processes. J Environ Manage 257:109973
Fu W et al (2022) When bimetallic oxides and their complexes meet Fenton-like process. J Hazardous Materials 424:127419
Garcia-Segura S, Brillas E, Cornejo-Ponce L, Salazar R (2016) Effect of the Fe3+/Cu2+ ratio on the removal of the recalcitrant oxalic and oxamic acids by electro-Fenton and solar photoelectro-Fenton. Sol Energy 124:242–253
Garcia-Segura S, Mostafa E, Baltruschat H (2017) Could NOx be released during mineralization of pollutants containing nitrogen by hydroxyl radical? Ascertaining the release of N-volatile species. Appl Catal B 207:376–384
Gligorovski S, Strekowski R, Barbati S, Vione D (2015) Environmental implications of hydroxyl radicals (•OH). Chem Rev 115(24):13051–13092
Haag WR, Hoigné J (1985) Photo-sensitized oxidation in natural water via •OH radicals. Chemosphere 14(11):1659–1671
Liu Y et al (2021) Polyoxometalate@Metal–Organic Framework Composites as Effective Photocatalysts. ACS Catal 11(21):13374–13396
Liu H, Cheng M, Liu Y, Zhang G, Li L, Du L, Li B, Xiao S, Wang G, Yang X (2022) Modified UiO-66 as photocatalysts for boosting the carbon-neutral energy cycle and solving environmental remediation issues. Coord Chem Rev 458:214428
Luna AJ, Nascimento CAO, Foletto EL, Moraes JEF, Chiavone-Filho O (2013) Photo-Fenton degradation of phenol, 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenol mixture in saline solution using a falling-film solar reactor. Environ Technol 35(3):364–371
Mafa PJ, Patala R, Mamba BB, Liu D, Gui J, Kuvarega AT (2020) Plasmonic Ag3PO4/EG photoanode for visible light-driven photoelectrocatalytic degradation of diuretic drug. Chem Eng J 393:124804
Moraes JEF, Quina FH, Nascimento CAO, Silva DN, Chiavone-Filho O (2004) Treatment of saline wastewater contaminated with hydrocarbons by the photo-Fenton process. Environ Sci Technol 38(4):1183–1187
Moreira FC, Boaventura RAR, Brillas E, Vilar VJP (2017) Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl Catal B 202:217–261
Mota ALN, Neto LGL, Foletto EL, Chiavone-Filho O, do Nascimento CAO (2018) Analysis of solar and artificial UVA irradiations on the photo-Fenton treatment of phenolic effluent and oilfield produced water. Chem Eng Commun 205(11):1594–1603
Mota-Lima A, Cunha-Filho FJV, Chiavone-Filho O, Nascimento CAO (2022) Efficiency of the H2O2 consumption by the mineralization of hydrochlorothiazide via photo-Fenton UVA: a time dependent analysis. Brazilian J Chem Eng Under Rev. https://doi.org/10.1007/s43153-022-00272-0
Murrieta MF, Sirés I, Brillas E, Nava JL (2020) Mineralization of Acid Red 1 azo dye by solar photoelectro-Fenton-like process using electrogenerated HClO and photoregenerated Fe(II). Chemosphere 246:125697
Nogueira RFP, Oliveira MC, Paterlini WC (2005) Simple and fast spectrophotometric determination of H2O2 in photo-Fenton reactions using metavanadate. Talanta 66(1):86–91
Oliveira MC, Nogueira RFP, Gomes Neto JA, Jardim WF, Rohwedder JJR (2001) Sistema de injeção em fluxo espectrofotométrico para monitorar peróxido de hidrogênio em processo de fotodegradação por reação foto-Fenton. Quim Nova 24:188–190
Paniagua CES, Amildon Ricardo I, Marson EO, Gonçalves BR, Trovó AG (2019) Simultaneous degradation of the pharmaceuticals gemfibrozil, hydrochlorothiazide and naproxen and toxicity changes during UV-C and UV-C/H2O2 processes in different aqueous matrixes. J Environ Chem Eng 7(3):103164
Real FJ, Acero JL, Benitez FJ, Roldán G, Fernández LC (2010) Oxidation of hydrochlorothiazide by UV radiation, hydroxyl radicals and ozone: Kinetics and elimination from water systems. Chem Eng J 160(1):72–78
Rodríguez EM, Márquez G, León EA, Álvarez PM, Amat AM, Beltrán FJ (2013) Mechanism considerations for photocatalytic oxidation, ozonation and photocatalytic ozonation of some pharmaceutical compounds in water. J Environ Manage 127:114–124
Schröder HF et al (2010) Anabolic, doping, and lifestyle drugs, and selected metabolites in wastewater—detection, quantification, and behaviour monitored by high-resolution MS and MSnbefore and after sewage treatment. Anal Bioanal Chem 398(3):1207–1229
Shi Q et al (2022) The application of transition metal-modified biochar in sulfate radical based advanced oxidation processes. Environ Res 212:113340
Vilar VJP, Alfonso-Muniozguren P, Monteiro JP, Lee J, Miranda SM, Boaventura RAR (2020) Tube-in-tube membrane microreactor for photochemical UVC/H2O2 processes: A proof of concept. Chem Eng J 379:122341
von Sonntag C (2007) The basics of oxidants in water treatment. Part A: OH radical reactions. Water Sci Technol 55(12):19–23
Wacławek S, Lutze HV, Grübel K, Padil VVT, Černík M, Dionysiou DD (2017) Chemistry of persulfates in water and wastewater treatment: A review. Chem Eng J 330:44–62
Yabalak E, Görmez Ö, Nural Y (2018) Mineralization of hydrochlorothiazide using hydrogen peroxide in subcritical water. J Turkish Chem Soc Sec A: Chem 5(3):1135–1144
Ye Z, Guelfi DRV, Álvarez G, Alcaide F, Brillas E, Sirés I (2019) Enhanced electrocatalytic production of H2O2 at Co-based air-diffusion cathodes for the photoelectro-Fenton treatment of bronopol. Appl Catal B 247:191–199
Funding
Graduate Program in Chemical Engineering of the Federal University of Rio Grande do Norte (UFRN) has funding the consumables; National Program for Academic Cooperation (PROCAD-CAPES, # 403230/2013–6) provide financial support to the scientists travel between the universities, and National Council of Research and Development (CNPq, #155046/2018–7) has support an individual scholarship.
Author information
Authors and Affiliations
Contributions
C.A.O.N. acquired funds; O. C-F administrated the project; A.M-L planned the experiments and conceptualized the methodology for data analysis; F.J.V.C-F employed different experimental techniques and methodologies to execute all the experiments; D.N.D. reproduced part of the experiments and confirmed the temperature elevation of the aqueous solution; A.M-L, D.N.S and F.J.V.C-F analyzed the data. A.M-L wrote the manuscript. C.A.O.N. revised the manuscript. All authors discussed the main results.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
All authors certify that they consent to participate in this research study.
Consent to publish
All authors certify that they give consent to publish this research study.
Competing interests
A.M-L, O. C-F, and F.J.V.C-F have no financial interests. Non-financial interests: Claudio A. Oller do Nascimento has served on advisory board Research Centre for Greenhouse Gas Innovation-RCGI.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cunha-Filho, F.J.V., do Nascimento Silva, D., do Nascimento, C.A.O. et al. Stoichiometric excesses of H2O2 as dosimetry strategy: proof of concept for UVC-H2O2, dark-Fenton, and UVC-Fenton. Environ Sci Pollut Res 30, 14860–14872 (2023). https://doi.org/10.1007/s11356-022-22968-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22968-z