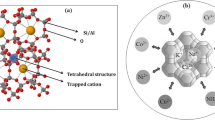
Abstract
The ability of clinoptilolite zeolite as a filter in water wells to remove lead from polluted groundwater was tested in batch and fixed-bed column experiments. XRF, XRD, SEM, and BET were used to characterize the zeolite. Because of the pH variation in groundwater, batch experiments were performed at pH = 6, 7, and 8, with the highest removal efficiency (84.2%) at pH = 6 and 298 K within 90 min. The Freundlich model accurately predicted metal ion adsorption behavior and indicated a multilayer adsorption of Pb(II) molecules on the inhomogeneous surface of clinoptilolite. The best-fitting kinetic model for clinoptilolite is the pseudo-second order equation, highlighting that the rate of adsorption is dependent on absorbent capacity. Next, the effect of flow rate, bed depth, and grain size of clinoptilolite on lead removal was investigated in column experiments at an initial concentration of 450 mg pb/L. The highest removal efficiency was achieved in column experiments with a flow rate of 1 mL/min, a bed height of 10 cm, and a grain size of 0.6 to 0.8 mm. Breakthrough curves were predicted by the Thomas and Yoon–Nelson models, with excellent agreement with the corresponding experimental data. This research will be used to develop a new in situ remedial approach for removing lead from polluted groundwater.












Similar content being viewed by others
Data availability
All data are provided in the manuscript.
References
Abusafa A, Yücel H (2002) Removal of 137Cs from aqueous solutions using different cationic forms of a natural zeolite: clinoptilolite. Sep Purif Technol 28(2):103–116
Adam MR, Othman MHD, Sheikh Abdul Kadir SH, Mohd Sokri MN, Tai ZS, Iwamoto Y, Tanemura M, Honda S, Puteh MH, Rahman MA (2020) Influence of the natural zeolite particle size toward the ammonia adsorption activity in ceramic hollow fiber membrane. Membranes 10(4):63
Adimalla N, Manne R, Zhang Y, Xu P, Qian H (2022) Evaluation of groundwater quality and its suitability for drinking purposes in semi-arid region of Southern India: An application of GIS. Geocarto International: 1–12
Aiban S, Znidarčić D (1989) Evaluation of the flow pump and constant head techniques for permeability measurements. Geotechnique 39(4):655–666
Al-Tabbaa A, Liska M, Ouellet-Plamondon C, Jegandan S, Shrestha R, Barker P, Critchlow C (2012) Soil mix technology for integrated remediation and ground improvement: from laboratory work to field trials. In Grouting and Deep Mixing, Louisiana, pp 522–532
Athman S, Sdiri A, Boufatit M (2020) Spectroscopic and mineralogical characterization of bentonite clay (Ghardaïa, Algeria) for heavy metals removal in aqueous solutions. Int J Environ Res 14(1):1–14
Ayotte P, Smith RS, Stevenson KP, Dohnálek Z, Kimmel GA, Kay BD (2001) Effect of porosity on the adsorption, desorption, trapping, and release of volatile gases by amorphous solid water. J Geophys Res Planets 106(E12):33387–33392
Bae Y-S, Yazaydın AOzr, Snurr RQ (2010) Evaluation of the BET method for determining surface areas of MOFs and zeolites that contain ultra-micropores. Langmuir 26(8):5475-5483
Bahiraei A, Behin J (2020) Sonochemical immobilization of MnO2 nanoparticles on NaP-zeolite for enhanced Hg (II) adsorption from water. J Environ Chem Eng 8(3):103790
Bandura L, Franus M, Józefaciuk G, Franus W (2015) Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 147:100–107
Banzhaf S, Hebig KH (2016) Use of column experiments to investigate the fate of organic micropollutants–a review. Hydrol Earth Syst Sci 20(9):3719–3737
Behin J, Ghadamnan E, Kazemian H (2019) Recent advances in natural zeolites sciences and technologies in Iran. Clay Miner 54:131–144
Benefield LD, Judkins JF, Weand BL (1982) Process chemistry for water and wastewater treatment. Prentice-Hall, Englewood Cliffs
Bertocchi AF, Ghiani M, Peretti R, Zucca A (2006) Red mud and fly ash for remediation of mine sites contaminated with As, Cd, Cu, Pb and Zn. J Hazard Mater 134(1–3):112–119
Bertoni FA, Medeot AC, González JC, Sala LF, Bellú SE (2015) Application of green seaweed biomass for MoVI sorption from contaminated waters. Kinetic, thermodynamic and continuous sorption studies. J Colloid Interface Sci 446:122–132
Breck D, Sieves ZM (1974) Structure chemistry and use. John Wiley and Sons, New York
Chakraborty R, Asthana A, Singh AK, Jain B, Susan ABH (2022) Adsorption of heavy metal ions by various low-cost adsorbents: a review. Int J Environ Anal Chem 102(2):342–379
Dokmen F, Kurtulus C (2008) Movement and flow velocity of groundwater in wells. Journal of Food, Agriculture & Environment 6(3–4):470–472
ElBastamy E, Ibrahim LA, Ghandour A, Zelenakova M, Vranayova Z, Abu-Hashim M (2021) Efficiency of natural clay mineral adsorbent filtration systems in wastewater treatment for potential irrigation purposes. Sustainability 13(10):5738
Fetter CW, Boving T, Kreamer D (2017) Contaminant hydrogeology, 3rd edn. Waveland Press Inc., Long Grove
Foroughi-dahr M, Esmaieli M, Abolghasemi H, Shojamoradi A, Sadeghi Pouya E (2016) Continuous adsorption study of Congo red using tea waste in a fixed-bed column. Desalin Water Treat 57(18):8437–8446
Fosso-Kankeu E, Waanders FB, Steyn FW (2017) Removal of Cr (VI) and Zn (II) from an aqueous solution using an organic-inorganic composite of bentonite-biochar-hematite. Desalination and Water Treatment 59:144–153
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57(1):385–470
Fu F, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205
Fuoco D (2012) A new method for characterization of natural zeolites and organic nanostructure using atomic force microscopy. Nanomaterials 2(1):79–91
Grant D, Mehdizadeh M, Chow A-L, Fairbrother J (1984) Non-linear van’t Hoff solubility-temperature plots and their pharmaceutical interpretation. Int J Pharm 18(1–2):25–38
Günay A, Arslankaya E, Tosun I (2007) Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics. J Hazard Mater 146(1–2):362–371
Han R, Zhang J, Zou W, Xiao H, Shi J, Liu H (2006) Biosorption of copper (II) and lead (II) from aqueous solution by chaff in a fixed-bed column. J Hazard Mater 133(1–3):262–268
Hao N, Ye J, Zhao L, Sun M, You Y, Zhang C, Cao J, Peng Y, Zhang S, Zhan L-T (2021) Evaluating iron remediation with limestone using spectral induced polarization and microscopic techniques. Sci Total Environ 800:149641
Hashim MA, Mukhopadhyay S, Sahu JN, Sengupta B (2011) Remediation technologies for heavy metal contaminated groundwater. J Environ Manage 92(10):2355–2388
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Ho Y, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76(4):332–340
Inglezakis VJ, Grigoropoulou HP (2003) Modeling of ion exchange of Pb2+ in fixed beds of clinoptilolite. Microporous Mesoporous Mater 61(1–3):273–282
International Association of Hydrogeologists (2020). Groundwater more about the hidden resource. Accessed 13 Nov 2020
Janani R, Gurunathan B, Sivakumar K, Varjani S, Ngo HH, Gnansounou E (2022) Advancements in heavy metals removal from effluents employing nano-adsorbents: way towards cleaner production. Environ Res 203:111815
Kabwadza-Corner P, Johan E, Matsue N (2015) pH dependence of lead adsorption on zeolites. J Environ Prot 6(01):45
Khajavian M, Salehi E, Vatanpour V (2020) Chitosan/polyvinyl alcohol thin membrane adsorbents modified with zeolitic imidazolate framework (ZIF-8) nanostructures: Batch adsorption and optimization. Sep Purif Technol 241:116759
Kumar PS, Korving L, Keesman KJ, van Loosdrecht MC, Witkamp G-J (2019) Effect of pore size distribution and particle size of porous metal oxides on phosphate adsorption capacity and kinetics. Chem Eng J 358:160–169
Kumar V, Dwivedi S, Oh S (2022) A critical review on lead removal from industrial wastewater: Recent advances and future outlook. J Water Process Eng 45:102518
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403
Levallois P, Barn P, Valcke M, Gauvin D, Kosatsky T (2018) Public health consequences of lead in drinking water. Curr Environ Health Rep 5(2):255–262
Li P (2020) To make the water safer. Expos Health 12(3):337–342
Li P, Wu J (2019) Drinking water quality and public health. Expos Health 11(2):73–79
Liu X, Pang H, Liu X, Li Q, Zhang N, Mao L, Qiu M, Hu B, Yang H, Wang X (2021) Orderly porous covalent organic frameworks-based materials: superior adsorbents for pollutants removal from aqueous solutions. The Innovation 2(1):100076
Mazloomi F, Jalali M (2016) Ammonium removal from aqueous solutions by natural Iranian zeolite in the presence of organic acids, cations and anions. J Environ Chem Eng 4(1):240–249
McCabe WL, Smith JC, Harriott P (1993) Unit operations of chemical engineering. Chemical and petroleum Engineering Series, 5th edn. McGraw-Hill International Edition, New York
Mckay G, Blair H, Gardner J (1982) Adsorption of dyes on chitin. I. Equilibrium studies. J Appl Polym Sci 27(8):3043–3057
Medvidović NV, Perić J, Trgo M, Mužek M (2006) Removal of lead ions by a fixed bed of clinoptilolite—the effect of the influent flow. 7th International Conference on the Occurrence, Properties, and Utilization of Natural Zeolites, 16–21 July , Socorro, New Mexico
Medvidović NV, Perić J, Trgo M, Mužek M (2007) Removal of lead ions by fixed bed of clinoptilolite–the effect of flow rate. Microporous Mesoporous Mater 105(3):298–304
Misaelides P (2011) Application of natural zeolites in environmental remediation: a short review. Microporous Mesoporous Mater 144(1–3):15–18
Missimer T, Lopez O (2018) Laboratory measurement of total porosity in unconsolidated quartz sand by two integrated methods. J Geol Geophys 7(448):2
Nakhaei M, Dadgar MA, Amiri V (2016) Geochemical processes analysis and evaluation of groundwater quality in Hamadan Province, Western Iran. Arab J Geosci 9(5):1–13
Newbury* DE, Ritchie NW (2013) Is scanning electron microscopy/energy dispersive X‐ray spectrometry (SEM/EDS) quantitative? Scanning 35(3):141-168
Obiri-Nyarko F, Grajales-Mesa SJ, Malina G (2014) An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 111:243–259
Osmari TA, Gallon R, Schwaab M, Barbosa-Coutinho E, Severo JB Jr, Pinto JC (2013) Statistical analysis of linear and non-linear regression for the estimation of adsorption isotherm parameters. Adsorpt Sci Technol 31(5):433–458
Pandey S, Fosso-Kankeu E, Spiro M, Waanders F, Kumar N, Ray SS, Kim J, Kang M (2020) Equilibrium, kinetic, and thermodynamic studies of lead ion adsorption from mine wastewater onto MoS2-clinoptilolite composite. Mater Today Chem 18:100376
Pansini M (1996) Natural zeolites as cation exchangers for environmental protection. Miner Deposita 31(6):563–575
Park J-B, Lee S-H, Lee J-W, Lee C-Y (2002) Lab scale experiments for permeable reactive barriers against contaminated groundwater with ammonium and heavy metals using clinoptilolite (01–29B). J Hazard Mater 95(1–2):65–79
Patel H (2019) Fixed-bed column adsorption study: a comprehensive review. Appl Water Sci 9(3):1–17
Pawluk K, Fronczyk J (2015) Evaluation of single and multilayered reactive zones for heavy metals removal from stormwater. Environ Technol 36(12):1576–1583
Perina T (2022) Semi-analytical model for solute transport in a three-dimensional aquifer with dual porosity and a volumetric source term. J Hydrol 607:127520
Qian G, Xu L, Li N, Wang K, Qu Y, Xu Y (2022) Enhanced arsenic migration in tailings soil with the addition of humic acid, fulvic acid and thiol-modified humic acid. Chemosphere 286:131784
Rakhym A, Seilkhanova G, Kurmanbayeva T (2020) Adsorption of lead (II) ions from water solutions with natural zeolite and chamotte clay. Mater Today: Proceedings 31:482–485
Sahoo TR, Prelot B (2020) Adsorption processes for the removal of contaminants from wastewater: the perspective role of nanomaterials and nanotechnology. Elsevier, Nanomaterials for the detection and removal of wastewater pollutants, pp 161–222
Salehi E, Madaeni S, Raad SS, Manesh AS, Vatanpour V (2011) Thermodynamically comparison of Na+ and Ca2+ adsorption onto PVD and NF45 membranes. Desalination 281:312–318
Speight JG (2019) Natural water remediation: chemistry and technology. Butterworth-Heinemann, Oxford (An imprint of Elsevier)
Štrkalj A, Malina J (2011) Thermodynamic and kinetic study of adsorption of Ni(II) ions on carbon anode dust. Chem Eng Commun 198(12):1497–1504
Taka AL, Fosso-Kankeu E, Pillay K, Mbianda XY (2018) Removal of cobalt and lead ions from wastewater samples using an insoluble nanosponge biopolymer composite: adsorption isotherm, kinetic, thermodynamic, and regeneration studies. Environ Sci Pollut Res 25(22):21752–21767
Todd DK, Mays LW (2004) Groundwater hydrology, 3rd edn. John Wiley & Sons, Inc., New York
Tran HN, You S-J, Hosseini-Bandegharaei A, Chao H-P (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116
Trgo M, Medvidović NV, Perić J (2011) Application of mathematical empirical models to dynamic removal of lead on natural zeolite clinoptilolite in a fixed bed column
Vukojević Medvidović N, Nuić I, Ugrina M, Trgo M (2018) Evaluation of natural zeolite as a material for permeable reactive barrier for remediation of zinc-contaminated groundwater based on column study. Water Air Soil Pollut 229(11):1–14
Wibowo E, Rokhmat M, Abdullah M (2017) Reduction of seawater salinity by natural zeolite (clinoptilolite): adsorption isotherms, thermodynamics and kinetics. Desalination 409:146–156
Yang X, Yang S, Yang S, Hu J, Tan X, Wang X (2011) Effect of pH, ionic strength and temperature on sorption of Pb(II) on NKF-6 zeolite studied by batch technique. Chem Eng J 168(1):86–93
Zhang Y, Wang F, Cao B, Yin H, Al-Tabbaa A (2022) Simultaneous removal of Pb and MTBE by mixed zeolites in fixed-bed column tests. J Environ Sci 122:41–49
Funding
This research was part of a PhD thesis conducted at Kharazmi University, Iran, and the financial support was received from Vice Presidency for Science and Technology, Islamic Republic of Iran.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by them. The first draft of the manuscript was written by them, and they commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
All authors made substantial contributions to the conception, design, data interpretation, and all the steps involved in this work.
Consent for publication
All authors have approved the version to be published.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakhaei, M., Heidarian, M.H., Vatanpour, V. et al. Evaluation the feasibility of using clinoptilolite as a gravel pack in water wells for removal of lead from contaminated groundwater. Environ Sci Pollut Res 30, 4653–4668 (2023). https://doi.org/10.1007/s11356-022-22519-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22519-6