Abstract
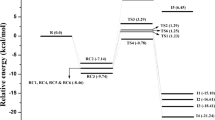
As one of the volatile organic compounds (VOCs) in the environment, 1,2,4,5-tetramethylbenzene (1,2,4,5-TeMB) present in oily wastewater, and it can occur substitution, abstraction, and addition reactions with OH radicals in the atmosphere and wastewater. Electrostatic potential (ESP) and average local ionization energy (ALIE) prediction indicate that H atoms from CH3 group and the benzene ring are the most active sites in 1,2,4,5-TeMB. The result shows that potential energy surfaces (PESs) in the gas and aqueous phase are similar, and the relevant barriers in the latter one are higher. The dominant channel is H abstraction from the benzene ring, and the subdominant one is OH radical addition to the benzene ring. Furthermore, subsequent reactions of dominant products with O2, NO2, NO, and OH radicals in the atmosphere are studied, as well. The total reaction rate constant is calculated to be 2.36×10−10 cm3 molecule−1 s−1 at 1 atm and 298 K in the atmosphere, which agrees well with the experimental data. While the total rate constant in the aqueous phase is much lower than that in the gas phase. Ecologic toxicity analysis shows that 1,2,4,5-TeMB is very toxic to fish, daphnia, and green algae; and OH-initiated degradation in the environment will reduce its toxicity.








Similar content being viewed by others
References
Alarcón P, Bohn B, Zetzsch C (2015) Kinetic and mechanistic study of the reaction of OH radicals with methylated benzenes: 1,4-dimethyl-, 1,3,5-trimethyl-, 1,2,4,5-, 1,2,3,5- and 1,2,3,4-tetramethyl-, pentamethyl-, and hexamethylbenzene. Phys Chem Chem Phys 17:13053–13065. https://doi.org/10.1039/C5CP00253B
Alarcón P, Bohn B, Berkemeier T et al (2021) Gas-phase reaction kinetics of the ortho and ipso adducts 1,2,4,5-tetramethylbenzene–OH with O2. ACS Earth Space Chem. https://doi.org/10.1021/acsearthspacechem.1c00230
Andreolli M, Lampis S, Brignoli P, Vallini G (2015) Bioaugmentation and biostimulation as strategies for the bioremediation of a burned woodland soil contaminated by toxic hydrocarbons: a comparative study. J Environ Manage 153:121–131. https://doi.org/10.1016/j.jenvman.2015.02.007
Andrews NLP, Fan JZ, Forward RL et al (2017) Determination of the thermal, oxidative and photochemical degradation rates of scintillator liquid by fluorescence EEM spectroscopy. Phys Chem Chem Phys 19:73–81. https://doi.org/10.1039/C6CP06015C
Arenas JF, Avila FJ, Otero JC et al (2008) Approach to the atmospheric chemistry of methyl nitrate and methylperoxy nitrite. Chemical mechanisms of their formation and decomposition reactions in the gas phase. J Phys Chem A 112:249–255. https://doi.org/10.1021/jp075546n
Aschmann SM, Arey J, Atkinson R (2013) Rate constants for the reactions of OH radicals with 1,2,4,5-tetramethylbenzene, pentamethylbenzene, 2,4,5-trimethylbenzaldehyde, 2,4,5-trimethylphenol, and 3-methyl-3-hexene-2,5-dione and products of OH + 1,2,4,5-tetramethylbenzene. J Phys Chem A 117:2556–2568. https://doi.org/10.1021/jp400323n
Bohn B, Zetzsch C (2012) Kinetics and mechanism of the reaction of OH with the trimethylbenzenes – experimental evidence for the formation of adduct isomers. Phys Chem Chem Phys 14:13933. https://doi.org/10.1039/C2CP42434G
Canneaux S, Bohr F, Henon E (2014) KiSThelP: a program to predict thermodynamic properties and rate constants from quantum chemistry results †. J Comput Chem 35:82–93. https://doi.org/10.1002/jcc.23470
Chen J, Qu R, Pan X, Wang Z (2016) Oxidative degradation of triclosan by potassium permanganate: kinetics, degradation products, reaction mechanism, and toxicity evaluation. Water Res 103:215–223. https://doi.org/10.1016/j.watres.2016.07.041
Davidson MM, Hillier IH, Hall RJ, Burton NA (1994) Modelling the reaction OH- + CO2 HCO-3 in the gas phase and in aqueous solution: a combined density functional continuum approach. Mol Phys 83:327–333. https://doi.org/10.1080/00268979400101281
Deacon GB, Forsyth CM, Junk PC et al (2008) Novel rare earth quinolinolate complexes. J Alloys Compd 451:436–439. https://doi.org/10.1016/j.jallcom.2007.04.253
Derwent RG, Jenkin ME, Saunders SM (1996) Photochemical ozone creation potentials for a large number of reactive hydrocarbons under European conditions. Atmos Environ. https://doi.org/10.1016/1352-2310(95)00303-G
Fitzky AC, Sandén H, Karl T et al (2019) The interplay between ozone and urban vegetation—BVOC emissions, ozone deposition, and tree ecophysiology. Front For Glob Change 2:50. https://doi.org/10.3389/ffgc.2019.00050
Frisch MJ, Trucks GW, Schlegel HB, et al (2009) Gaussian 09, Revision A.1
Frka S, Šala M, Kroflič A et al (2016) Quantum chemical calculations resolved identification of methylnitrocatechols in atmospheric aerosols. Environ Sci Technol 50:5526–5535. https://doi.org/10.1021/acs.est.6b00823
Fukui K (1970) A formulation of the reaction coordinate. https://doi.org/10.1021/j100717a029
Fukui K (1975) Chemical reactivity theory. In: Theory of orientation and stereoselection. Springer, Berlin Heidelberg, pp 8–9
Guenther AB, Jiang X, Heald CL et al (2012) The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci Model Dev 5:1471–1492. https://doi.org/10.5194/gmd-5-1471-2012
Homlok R, Mile V, Takács E et al (2020) Comparison of hydrogen atom and hydroxyl radical reactions with simple aromatic molecules in aqueous solution. Chem Phys 534:110754. https://doi.org/10.1016/j.chemphys.2020.110754
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138. https://doi.org/10.1103/PhysRev.140.A1133
Kukkadapu G, Kang D, Wagnon SW et al (2019) Kinetic modeling study of surrogate components for gasoline, jet and diesel fuels: C7-C11 methylated aromatics. Proc Combust Inst 37:521–529. https://doi.org/10.1016/j.proci.2018.08.016
Kumar PGA (2006) PGSE diffusion NMR—an emerging technique for inorganic/organometallic chemists. Aust J Chem 59:78. https://doi.org/10.1071/CH05251
Launder AM, Agarwal J, Schaefer HF (2015) Exploring mechanisms of a tropospheric archetype: CH3O2 + NO. J Chem Phys 143:234302. https://doi.org/10.1063/1.4937381
Lawrence MG, Jockel P (2001) What does the global mean OH concentration tell us? Atmos Chem Phys 13. https://doi.org/10.5194/acp-1-37-2001
Li Y, Wang L (2014) The atmospheric oxidation mechanism of 1,2,4-trimethylbenzene initiated by OH radicals. Phys Chem Chem Phys 16:17908. https://doi.org/10.1039/C4CP02027H
Li Y, Zhong Q (2009) The characterization and activity of F-doped vanadia/titania for the selective catalytic reduction of NO with NH3 at low temperatures. J Hazard Mater 172:635–640. https://doi.org/10.1016/j.jhazmat.2009.07.039
Li W, Yu H, Zhang Z et al (2021) Electrochemical removal of NOx by La0.8Sr0.2Mn1−xNixO3 electrodes in solid electrolyte cells: role of Ni substitution. J Hazard Mater 420:126640. https://doi.org/10.1016/j.jhazmat.2021.126640
Liu Q-X, Zhao X-J, Wu X-M et al (2008) Two new N-heterocyclic carbene silver(I) complexes with the π–π stacking interactions. Inorganica Chim Acta 361:2616–2622. https://doi.org/10.1016/j.ica.2007.11.008
Miertuš S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem Phys 55:117–129. https://doi.org/10.1016/0301-0104(81)85090-2
Mokhbi Y, Korichi M, Akchiche Z (2019) Combined photocatalytic and Fenton oxidation for oily wastewater treatment. Appl Water Sci 9:35. https://doi.org/10.1007/s13201-019-0916-x
Mouchel-Vallon C, Bräuer P, Camredon M et al (2013) Explicit modeling of volatile organic compounds partitioning in the atmospheric aqueous phase. Atmospheric Chem Phys 13:1023–1037. https://doi.org/10.5194/acp-13-1023-2013
Nguyen HT, Mai TV-T, Huynh LK (2017) Detailed kinetic mechanism for CH3OO + NO reaction – an ab initio study. Comput Theor Chem 1113:14–23. https://doi.org/10.1016/j.comptc.2017.04.015
Piccot SD, Watson JJ, Jones JW (1992) A global inventory of volatile organic compound emissions from anthropogenic sources. J Geophys Res Atmospheres 97:9897–9912. https://doi.org/10.1029/92JD00682
Ponnusamy S, Sandhiya L, Senthilkumar K (2017) The atmospheric oxidation mechanism and kinetics of 1,3,5-trimethylbenzene initiated by OH radicals – a theoretical study. New J Chem 41:10259–10271. https://doi.org/10.1039/C7NJ01285C
Qu R, Liu J, Li C et al (2016) Experimental and theoretical insights into the photochemical decomposition of environmentally persistent perfluorocarboxylic acids. Water Res 104:34–43. https://doi.org/10.1016/j.watres.2016.07.071
Qu R, Li C, Pan X et al (2017) Solid surface-mediated photochemical transformation of decabromodiphenyl ether (BDE-209) in aqueous solution. Water Res 125:114–122. https://doi.org/10.1016/j.watres.2017.08.033
Qu R, Li C, Liu J et al (2018) Hydroxyl radical based photocatalytic degradation of halogenated organic contaminants and paraffin on silica gel. Environ Sci Technol 52:7220–7229. https://doi.org/10.1021/acs.est.8b00499
Rao Z, Lu G, Chen L et al (2022) Photocatalytic oxidation mechanism of gas-phase VOCs: unveiling the role of holes, center dot OH and center dot O-2(-). Chem Eng J 430:132766. https://doi.org/10.1016/j.cej.2021.132766
Roy J, Ojha PK, Carnesecchi E et al (2020) First report on a classification-based QSAR model for chemical toxicity to earthworm. J Hazard Mater 386:121660. https://doi.org/10.1016/j.jhazmat.2019.121660
Scheiner S (2012) Evaluation of DFT methods to study reactions of benzene with OH radical: evaluation of DFT Methods. Int J Quantum Chem 112:1879–1886. https://doi.org/10.1002/qua.23089
Seeger ZL, Izgorodina EI (2020) A systematic study of DFT performance for geometry optimizations of ionic liquid clusters. J Chem Theory Comput 16:6735–6753. https://doi.org/10.1021/acs.jctc.0c00549
Shen X, Zhao Y, Chen Z, Huang D (2013) Heterogeneous reactions of volatile organic compounds in the atmosphere. Atmos Environ 68:297–314. https://doi.org/10.1016/j.atmosenv.2012.11.027
Stimac PJ, Barker JR (2008) Non-RRKM dynamics in the CH3O2 + NO reaction system. J Phys Chem A 112:2553–2562. https://doi.org/10.1039/C5RA06732D
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094. https://doi.org/10.1021/cr9904009
US EPA O (2015) Ecological Structure Activity Relationships (ECOSAR) predictive model. https://www.epa.gov/tsca-screening-tools/ecological-structure-activity-relationships-ecosar-predictive-model. Accessed 10 Nov 2021
Velasco E, Lamb B, Westberg H et al (2007) Distribution, magnitudes, reactivities, ratios and diurnal patterns of volatile organic compounds in the Valley of Mexico during the MCMA 2002 & 2003 field campaigns. Atmospheric Chem Phys 7:329–353. https://doi.org/10.5194/acp-7-329-2007
Warneke C (2004) Comparison of daytime and nighttime oxidation of biogenic and anthropogenic VOCs along the New England coast in summer during New England Air Quality Study 2002. J Geophys Res 109:D10309. https://doi.org/10.1029/2003JD004424
Wigner, E (1932) ber das berschreiten von Potentialschwellen bei chemischen Reaktionen. Z Für Phys Chem 19B. https://doi.org/10.1007/978-3-642-59033-7_8
Wijesiri B, Liu A, Hong N et al (2019) Rethinking hydrocarbons build-up on urban roads: a perspective on volatilisation under global warming scenarios. Environ Pollut 252:950–959. https://doi.org/10.1016/j.envpol.2019.06.044
Xia L, Cai C, Zhu B et al (2014) Source apportionment of VOCs in a suburb of Nanjing, China, in autumn and winter. J Atmospheric Chem 71:175–193. https://doi.org/10.1007/s10874-014-9289-6
Zhang S, Huang H, Su J et al (2014) Geochemistry of alkylbenzenes in the Paleozoic oils from the Tarim Basin, NW China. Org Geochem 77:126–139. https://doi.org/10.1016/j.orggeochem.2014.10.003
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Zhao H, Lu C, Tang Y et al (2022) A theoretical investigation on the degradation reactions of CH3CH2CH2NH and (CH3CH2CH2)2N radicals in the presence of NO, NO2 and O2. Chemosphere 287:131946. https://doi.org/10.1016/j.chemosphere.2021.131946
Zhu L, Shen D, Luo KH (2020) A critical review on VOCs adsorption by different porous materials: species, mechanisms and modification methods. J Hazard Mater 389:122102. https://doi.org/10.1016/j.jhazmat.2020.122102
Availability of data
The data that support the findings of this study are attached.
Funding
This work has been supported by the National Natural Science Foundation of China (41775119), Focuses on Research and Development Plan in Shandong Province (2019GSF109046), and Natural Science Foundation of Shandong Province (ZR2021MB104).
Author information
Authors and Affiliations
Contributions
H. Zhao, S.J. Wang, and C.G. Lu performed electronic structure and kinetics calculations in this manuscript. Y.Z. Tang wrote the manuscript. J.Y. Sun, Y.J. Zhang, J. Guan, and Y.R. Pan helped in discussing and revising the manuscript. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1324 kb)
Rights and permissions
About this article
Cite this article
Zhao, H., Sun, J., Zhang, Y. et al. Investigations on mechanisms, kinetics, and ecotoxicity in OH-initiated degradation of 1,2,4,5-tetramethylbenzene in the environment. Environ Sci Pollut Res 29, 84616–84628 (2022). https://doi.org/10.1007/s11356-022-21704-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21704-x