Abstract
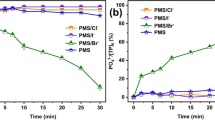
Advanced oxidation processes (AOPs) are efficient methods for water purification. However, there are few studies on using peroxymonosulfate (PMS) to remove pollutants directly. In this study, about 76% of methylene blue (MB) was removed by PMS directly within 180 min through a non-radical pathway, verified by scavenging tests, electron paramagnetic resonance and kinetic calculations. Additionally, the effects of PMS dosage, MB concentration, temperature, initial pH and competitive anions were determined. High PMS dosage, temperature and pH promoted MB degradation (from 76 to 98%) while MB concentration showed no effect on MB removal. Besides, MB degradation followed pseudo-first-order kinetic with rate constants of 0.0082 to 0.3912 min−1. The second-order rate constant for PMS reaction with MB was 0.08 M−1 s−1 at pH 3–6, but increased dramatically to 4.68 M−1 s−1 at pH 10.50. Chlorine could be catalysed by PMS at high concentration Cl− and degradation efficiency reached 98% within 90 min. High concentration of bicarbonate accelerated MB removal due to the high pH value while humic acid showed a marginal effect on MB degradation. Furthermore, TOC removal rate of MB in the presence of chloride reached 45%, whereas PMS alone caused almost no mineralisation. This study provides new insights into pollutant removal and an additional strategy for water purification.






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
Ahmad M, Teel A, Watts R (2013) Mechanism of persulfate activation by phenols. Environ Sci Technol 47:5864–5871
Bohme K, Brauer H (1992) Generation of singlet oxygen from hydrogen peroxide disproportionation catalyzed by molybdate ions. Inorg Chem 31:3468–3471
Chen L, Zuo X, Yang S, Cai T, Ding D (2019a) Rational design and synthesis of hollow Co3O4@Fe2O3 core-shell nanostructure for the catalytic degradation of norfloxacin by coupling with peroxymonosulfate. Chem Eng J 359:373–384
Chen X, Oh W, Lim T (2018) Graphene- and CNTs-based carbocatalysts in persulfates activation: material design and catalytic mechanisms. Chem Eng J 354:941–976
Chen X, Yang B, Oleszczuk P, Gao Y, Yuan X, Ling W, Waigi M (2019b) Vanadium oxide activates persulfate for degradation of polycyclic aromatic hydrocarbons in aqueous system. Chem Eng J 364:79–88
Chen Z, Wan Q, Wen G, Luo X, Xu X, Wang J, Li K, Huang T, Ma J (2021) Effect of borate buffer on organics degradation with unactivated peroxymonosulfate: influencing factors and mechanisms. Sep Purif Technol 256:117841
Cheng X, Liang H, Ding A, Qu F, Shao S, Liu B, Wang H, Wu D, Li G (2016) Effects of pre-ozonation on the ultrafiltration of different natural organic matter (NOM) fractions: membrane fouling mitigation, prediction and mechanism. J Membr Sci 505(1):15–25
Connick R, Zhang Y, Lee S, Adamic P (1995) Kinetics and mechanism of the oxidation of HSO3- by O2. 1. The Uncatalyzed Reaction Inorganic Chemistry 34:4543–4553
Deborde M, von Gunten U (2008) Reactions of chlorine with inorganic and organic compounds during water treatment-kinetics and mechanisms: a critical review. Water Res 42(1–2):13–51
Ding D, Liu C, Ji Y, Yang Q, Chen L, Jiang C, Cai T (2017) Mechanism insight of degradation of norfloxacin by magnetite nanoparticles activated persulfate: identification of radicals and degradation pathway. Chem Eng J 308:330–339
Ding J, Nie H, Wang S, Chen Y, Wan Y, Wang J, Xiao H, Yue S, Ma J, Xie P (2021) Transformation of acetaminophen in solution containing both peroxymonosulfate and chlorine: performance, mechanism, and disinfection by-product formation. Water Res 189:116605
Espinosa J, Manickam-Periyaraman P, Bernat-Quesada F, Sivanesan S, Álvaro M, García H, Navalón S (2019) Engineering of activated carbon surface to enhance the catalytic activity of supported cobalt oxide nanoparticles in peroxymonosulfate activation. Appl Catal B 249:42–53
Feng M, Baum J, Nesnas N, Lee Y, Huang C, Sharma V (2019) Oxidation of sulfonamide antibiotics of six-membered heterocyclic moiety by ferrate(VI): kinetics and mechanistic insight into SO2 extrusion. Environ Sci Technol 53(5):2695–2704
Guan Y, Ma J, Ren Y, Liu Y, Xiao J, Lin L, Zhang C (2013) Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res 47(14):5431–5438
He X, Mzeyk S, Michael I, Fatta-Kassinos D, Dionysiou D (2014) Degradation kinetics and mechanism of beta-lactam antibiotics by the activation of H2O2 and Na2S2O8 under UV-254 nm irradiation. J Hazard Mater 279:375–383
Huang D, Wang T, Zhu K, Zhao S, Shi Y, Ye M, Wang C, Jia H (2019) Low-molecular-weight organic acids impede the degradation of naphthol in iron oxides/persulfate system: Implications for research experiments in pure conditions. Chemosphere 225:1–8
Li C, Wang Y, Chen C, Fu X, Cui S, Lu J, Liu H, Li W (2019) Interactions between chlorophenols and peroxymonosulfate: pH dependency and reaction pathways. Sci Total Environ 664:133–139
Liao X, Cao J, Hu Y, Zhang C, Hu L (2021) Mechanism of unactivated peroxymonosulfate-induced degradation of methyl parathion: kinetics and transformation pathway. Chemosphere 284:131332
Long X, Yang S, Qiu X, Ding D, Feng C, Chen R, Tan J, Wang X, Chen N, Lei Q (2021) Heterogeneous activation of peroxymonosulfate for bisphenol A degradation using CoFe2O4 derived by hybrid cobalt-ion hexacyanoferrate nanoparticles. Chem Eng J 404:127052
Lu J, Ji Y, Chovelon J, Lu J (2021) Fluoroquinolone antibiotics sensitized photodegradation of isoproturon. Water Res 198:117136
Mohamed F, Allah P, Mehdi A, Baseem M (2011) Photoremoval of Malachite Green (MG) using advanced oxidation process. Res J Chem Environ 15(3):65–70
Monteagudo J, El-Taliawy H, Duran A, Caro G, Bester K (2018) Sono-activated persulfate oxidation of diclofenac: degradation, kinetics, pathway and contribution of the different radicals involved. J Hazard Mater 357:457–465
Mussa Z, Othman M, Abdullah M, Nordin N (2013) Decolorization of landfill leachate using electrochemical technique. Int J Chem Sci 11(4):1636–1646
Javier F, Solís R (2018) Chloride promoted oxidation of tritosulfuron by peroxymonosulfate. Chem Eng J 349:728–736
Ji Y, Lu J, Wang L, Jiang M, Yang Y, Yang P, Zhou L, Ferronato C, Chovelon J (2018) Non-activated peroxymonosulfate oxidation of sulfonamide antibiotics in water: kinetics, mechanisms, and implications for water treatment. Water Res 147:82–90
Pang Y, Tong Z, Tang L, Liu Y, Luo K (2018) Effect of humic acid on the degradation of methylene blue by peroxymonosulfate. Open Chem 16(1):401–406
Peng Q, Ding Y, Zhu L, Zhang G, Tang H (2018) Fast and complete degradation of norfloxacin by using Fe/Fe3C@NG as a bifunctional catalyst for activating peroxymonosulfate. Sep Purif Technol 202:307–317
Qi C, Liu X, Li Y, Li C, Ma J, Li X, Zhang H (2017) Enhanced degradation of organic contaminants in water by peroxydisulfate coupled with bisulfate. J Hazard Mater 328:98–107
Qi C, Liu X, Ma J, Lin C, Li X, Zhang H (2016) Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 151:280–288
Qiang Z, Adams C (2004) Determination of monochloramine formation rate constants with stopped-flow spectrophotometry. Environ Sci Technol 38:1435–1444
Shimizu N, Ogino C, Dadjour M, Murata T (2007) Sonocatalytic degradation of methylene blue with TiO2 pellets in water. Ultrason Sonochem 14:184–190
Song W, Li J, Fu C, Wang Z, Zhang X, Yang J, William H, Gao L (2019) Kinetics and pathway of atrazine degradation by a novel method: persulfate coupled with dithionite. Chem Eng J 373:803–813
Sun M, Huang W, Cheng H, Ma J, Kong Y, Komarneni S (2019) Degradation of dye in wastewater by homogeneous Fe(VI)/NaHSO3 system. Chemosphere 228:595–601
Sun Y, Xie H, Zhou C, Wu Y, Pu M, Niu J (2021) The role of carbonate in sulfamethoxazole degradation by peroxymonosulfate without catalyst and the generation of carbonate radicals. J Hazard Mater 398:122827
Tan C, Gao N, Deng Y, Li L, Deng J, Zhou S (2015) Kinetics oxidation of antipyrine in heat-activated persulfate. Desalin Water Treat 53(1):263–271
Wang J, Duan X, Gao J, Shen Y, Feng X, Yu Z, Tan X, Liu S, Wang S (2020) Roles of structure defect, oxygen groups and heteroatom doping on carbon in nonradical oxidation of water contaminants. Water Res 185:116244
Wang Z, Yuan R, Guo Y, Xu L, Liu J (2011) Effects of chloride ions on bleaching of azo dyes by Co2+/oxone regent: kinetic analysis. J Hazard Mater 190(1–3):1083–1087
Wu S, Liu H, Yang C, Li X, Lin Y, Yin K, Sun J, Teng Q, Du C, Zhong Y (2020) High-performance porous carbon catalysts doped by iron and nitrogen for degradation of bisphenol F via peroxymonosulfate activation. Chem Eng J 392:123683
Wu S, Shen L, Lin Y, Yin K, Yang C (2021) Sulfite-based advanced oxidation and reduction processes for water treatment. Chem Eng J 414:128872
Wu S, Yang C, Lin Y, Cheng J (2022) Efficient degradation of tetracycline by singlet oxygen-dominated peroxymonosulfate activation with magnetic nitrogen-doped porous carbon. J Environ Sci 115:330–340
Xie P, Ma J, Liu W, Zhou J, Yue S, Li X, Wisner M, Fang J (2015) Removal of 2-MIB and geosmin using UV/persulfate: contribution of hydroxyl and sulfate radicals. Water Res 69:223–233
Xu H, Meng L, Zhao X, Chen J, Lu J, Chovelon J (2021) Accelerated oxidation of the emerging brominated flame retardant tetrabromobisphenol S by unactivated peroxymonosulfate: the role of bromine catalysis and formation of disinfection byproducts. Water Res 204:117584
Yang Y, Banerjee G, Brudvig G, Kim J, Pignatello J (2018a) Oxidation of organic compounds in water by unactivated peroxymonosulfate. Environ Sci Technol 52(10):5911–5919
Yang X, Ding X, Zhou L, Zhao Q, Ji Y, Wang X, Chovelon J, Xiu G (2021a) Direct oxidation of antibiotic trimethoprim by unactivated peroxymonosulfate via a nonradical transformation mechanism. Chemosphere 263:128194
Yang F, Huang Y, Fang C, Xue Y, Ai L, Liu J, Wang Z (2018b) Peroxymonosulfate/base process in saline wastewater treatment: the fight between alkalinity and chloride ions. Chemosphere 199:84–88
Yang B, Luo Q, Li Q, Meng Y, Li L, Liu Y (2021b) Selective oxidation and direct decolorization of cationic dyes by persulfate without activation. Water Sci Technol 83(11):2744–2752
Yao T, Wang J, Xue Y, Yu W, Gao Q, Ferreira L, Ren K, Ji J (2019) A photodynamic antibacteria spray-coating based on the host-guest immobilization of the photosensitizer methylene blue. J Mater Chem B 7(33):5089–5095
Yuan R, Ramjaun S, Wang Z, Liu J (2011) Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process: implications for formation of chlorinated aromatic compounds. J Hazard Mater 196:173–179
Zhou H, Lai L, Wan Y, He Y, Yao G, Lai B (2020a) Molybdenum disulfate(MoS2): a versatile activation of both peroxymonosulfate and persulfate for the degradation of carbamazepine. Chem Eng J 384:123264
Zhou Y, Gao Y, Jian J, Shen Y, Pang S, Wang Z, Duan J, Guo Q, Guan C, Ma J (2020b) Transformation of tetracycline antibiotics during water treatment with unactivated peroxymonosulfate. Chem Eng J 379:122378
Zhou Y, Jiang J, Gao Y, Pang S, Yang Y, Ma J, Gu J, Li J, Wang Z, Wang L, Yuan L, Yang Y (2017) Activation of peroxymonosulfate by phenols: important role of quinone intermediates and involvement of singlet oxygen. Water Res 125:209–218
Funding
This work was supported by the National Military Standard Research Project of China (20CH0612) and Dazhangxing Project of Naval Medical Center of PLA (21M0601).
Author information
Authors and Affiliations
Contributions
Xu Zuo: conceptualization, investigation, writing—original draft. Jianxin Nie: writing—review and editing. Beier Jiang: editing. Aijun Jiang: methodology. Shiyang Zou: editing and supervision. Junrong Wu: supervision. Bingquan Ding: investigation. Xuehui Wang: validation. Yang Liu: investigation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
The authors all agree to participate in the paper.
Consent to publish
The authors all agree that the paper is published in the journal.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zuo, X., Nie, J., Jiang, B. et al. Direct degradation of methylene blue by unactivated peroxymonosulfate: reaction parameters, kinetics, and mechanism. Environ Sci Pollut Res 29, 75597–75608 (2022). https://doi.org/10.1007/s11356-022-21197-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21197-8