Abstract
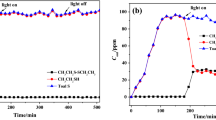
Ethyl mercaptans which commonly exist in natural gas need to be removed due to their toxic, odorous, and corrosive properties. Herein, a novel Fe2O3-modified HNbMoO6 nanosheet catalyst (Fe2O3@e-HNbMoO6) was prepared by an exfoliation-impregnation method for the ethyl mercaptans removal. In the heterojunction catalyst, e-HNbMoO6 can be excited by visible light to generate the photogenic charge and has certain adsorption property for ethyl mercaptan with hydrogen bonding (Nb-OH or Mo-OH as the hydrogen bonding donor); Fe2O3 plays the role of accelerating photogenerated electrons and holes, and enhancing the adsorption of ethyl mercaptan with another hydrogen bonding (Fe-OH as the hydrogen bonding donor and receptor). Results showed that the adsorption capacity of Fe2O3@e-HNbMoO6 is 69.9 μmol/g for ethyl mercaptan. In addition, the photocatalytic conversion efficiency of ethyl mercaptan to diethyl disulfide is nearly 100% and it is higher than that of the other Nb-Mo based photocatalysts, such as LiNbMoO6, Fe1/3NbMoO6, Ce1/3NbMoO6, TiO2-HNbMoO6, e-HNbMoO6, CeO2@e-HNbMoO6, and Ag2O@e-HNbMoO6. Under the experimental conditions, the photocatalytic conversion efficiency is greater than the adsorption efficiency over Fe2O3@e-HNbMoO6, and there is no ethyl mercaptan output in the process of adsorption and photocatalytic conversion. Fe2O3@e-HNbMoO6 heterojunction catalyst has practical value and reference significance for purifying methane gas and enhancing photocatalytic conversion of ethyl mercaptan.















Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Barzamini R, Falamaki C, Mahmoudi R (2014) Adsorption of ethyl, iso-propyl, n-butyl and iso-butyl mercaptans on AgX zeolite: equilibrium and kinetic study [J]. Fuel 130:46–53. https://doi.org/10.1016/j.fuel.2014.04.013
Bhuvaneswari R, Nagarajan V, Chandiramouli R (2020) Methyl and Ethyl mercaptan molecular adsorption studies on novel Kagome arsenene nanosheets - A DFT outlook. Physica B Condensed Matter 586:412135. https://doi.org/10.1016/j.physb.2020.412135
Ganiyu SA, Lateef SA (2021) Review of adsorptive desulfurization process: overview of the non-carbonaceous materials, mechanism and synthesis strategies. Fuel 294:120273. https://doi.org/10.1016/j.fuel.2021.120273
Garces HF, Espinal AE, Suib SL (2012) Tunable shape microwave synthesis of zinc oxide nanospheres and their desulfurization performance compared with nanorods and platelet-like morphologies for the removal of hydrogen sulfide. J. Phys. Chem. C 116:8465–8474. https://doi.org/10.1021/jp210755t
Garcia CL, Lercher JA (1993) Hydrogen bonding of sulfur containing compounds adsorbed on zeolite HZSM5. J. Mol. Struct. 293:235–238. https://doi.org/10.1016/0022-2860(93)80057-3
Greathouse JA, Hart DB, Ochs ME (2012) Alcohol and thiol adsorption on (oxy)hydroxide and carbon surfaces: molecular dynamics simulation and desorption experiments. J. Phys. Chem. C 116:26756–26764. https://doi.org/10.1021/jp305275q
Grimm OC, Somaratne R, Wang Y, Kim S, Whitten JE (2021) Thiol adsorption on metal oxide nanoparticles. Phys. Chem. Chem. Phys. 23:8309–8317. https://doi.org/10.1039/D1CP00506E
Guo CL, Zhu JC, He J, Hu LF, Zhang PP, Li DW (2020) Catalytic oxidation/photocatalytic degradation of ethyl mercaptan on α-MnO2@H4Nb6O17-NS nanocomposite. Vacuum 182:109718. https://doi.org/10.1016/j.vacuum.2020.109718
He J, Hu LF, Tang Y, Li HZ, Yang P, Li Z (2014) Adsorption features and photocatalytic oxidation performance of M1/3NbMoO6 (M = Fe, Ce) for ethyl mercaptan. RSC Adv. 4:22334–22341. https://doi.org/10.1039/C4RA01482K
Hosseinpour V, Kazemeini M, Mohammadi A, Rashidi A (2021) Design, manufacture and application of a microreactor for the decomposition of ethyl mercaptan on an H-ZSM-5 catalyst. J. Clean. Prod. 292:126036. https://doi.org/10.1016/j.jclepro.2021.126036
Hu LF, He J, Xu L, Li DW, Zhang PP (2016) Titania species on two-dimensional HNbMoO6 nanosheets: structural feature, interaction model, and synergistic effect for photocatalytic degradation of methylene blue. J. Nanophotonics 10:046015. https://doi.org/10.1117/1.JNP.10.046015
Hu LF, Zhu JC, Da LG, He J (2018) Effect of Ti species dosage on the photocatalytic performance of HNbMoO6. Appl. Phys. A 124:298. https://doi.org/10.1007/s00339-018-1728-9
Jafari L, Moradi H, Tavan Y (2020) A theoretical and industrial study of component co-adsorption on 3A zeolite: an industrial case. Chem. Pap. 74:651–661. https://doi.org/10.1007/s11696-019-00910-x
Khalkhali M, Ghorbani A, Bayati B (2019) Study of adsorption and diffusion of methyl mercaptan and methane on FAU zeolite using molecular simulation. Polyhedron 171:403–410. https://doi.org/10.1016/j.poly.2019.07.038
Liu J, Li C, Kong W, Lu Q, Zhang J, Qian GR (2020) Lactone radical transformed methyl mercaptan-adsorbed activated carbon into graphene oxide modified activated carbon. J. Hazard. Mater. 413:124527. https://doi.org/10.1016/j.jhazmat.2020.124527
Lv LD, Zhang J, Huang CP, Lei ZG, Chen BH (2014) Adsorptive separation of dimethyl disulfide from liquefied petroleum gas by different zeolites and selectivity study via FT-IR. Sep. Purif. Technol. 125:247–255. https://doi.org/10.1016/j.seppur.2014.02.002
Lyu Y, Liu X, Liu W, Tian YP, Qin ZG (2020) Adsorption/oxidation of ethyl mercaptan on Fe-N-modified active carbon catalyst. Chem. Eng. J. 393:124680. https://doi.org/10.1016/j.cej.2020.124680
Ma X, Liu HD, Li WM, Peng SP, Chen YF (2016) Reactive adsorption of low concentration methyl mercaptan on a Cu-based MOF with controllable size and shape. RSC Adv. 6:96997–97003. https://doi.org/10.1039/C6RA18593B
Ndagijimana P, Liu XJ, Li ZW, Xing ZJ, Pan BB, Yu GW, Wang Y (2021) Adsorption performance and mechanisms of mercaptans removal from gasoline oil using core-shell AC-based adsorbents. Environ. Sci. Pollut. Res. https://doi.org/10.1007/s11356-021-15075-y
Shen ZB, Ke M, Yu P, Hua HQ, Song ZZ, Jiang QZ (2015) Reaction mechanisms of thioetherification for mercaptans and olefins over sulfided Mo-Ni/Al2O3 catalysts. J. Mol. Catal A-Chem. 396:120–127. https://doi.org/10.1016/j.molcata.2014.09.034
Taheri A, Akhani EG, Towfighi J (2017) Methyl mercaptan removal from natural gas using MIL-53(Al). J. Nat. Gas Sci. Eng. 38:272–282. https://doi.org/10.1016/j.jngse.2016.12.029
Vinek H, Noller H, Ebel M, Schwarz K (1977) X-ray photoelectron spectroscopy and heterogeneous catalysis, with elimination reactions as an example. J. Chem. Soc. Faraday Trans. 73:734–746. https://doi.org/10.1039/F19777300734
Wang Y, Wang SL, Wu YB, Wang ZN, Zhang HH, Cao ZS, He J, Li W, Yang ZC, Zheng LC, Feng DQ, Pan P, Bi JL, Li HY, Zhao JS, Zhang KL (2020) A α-Fe2O3/rGO magnetic photocatalyst: enhanced photocatalytic performance regulated by magnetic field. J. Alloys Compd. 851:156733. https://doi.org/10.1016/j.jallcom.2020.156733
Wu WM, Lin R, Shen LJ, Liang RW, Yuan RS, Wu L (2013) Mechanistic insight into the photocatalytic hydrogenation of 4-nitroaniline over band-gap-tunable CdS photocatalysts. Phys. Chem. Chem. Phys. 15:19422–19426. https://doi.org/10.1039/C3CP53195C
Yi HH, Tao T, Zhao SZ, Yu QJ, Gao FY, Zhou YS, Tang XL (2020) Promoted adsorption of methyl mercaptan by γ-Al2O3 catalyst loaded with Cu/Mn. Environ. Technol. Inno. 21:101349. https://doi.org/10.1016/j.eti.2020.101349
Zhang JS, Chen XF, Takanabe K, Maeda K, Domen K, Epping JD, Fu XZ, Antonietti M, Wang XC (2010) Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew Chem Int Ed 49:441–444. https://doi.org/10.1002/anie.200903886
Zhang C, Wang Y, Zhang X, Wang RX, Kou LF, Li R, Fan CM (2020a) Preoxidation-assisted nitrogen enrichment strategy to decorate porous carbon spheres for catalytic adsorption/oxidation of methyl mercaptan. RSC Adv 10:37644–37656. https://doi.org/10.1039/D0RA07375J
Zhang W, Zhu JC, He J, Xu L, Hu LF (2020b) Construction of NiO/H2Ti3O7 nanotube composite and its photocatalytic conversion feature for ethyl mercaptan. Appl Phys A 126:630. https://doi.org/10.1007/s00339-020-03815-9
Zhang X, Wang LP, Hu LF, He J (2021) Adsorption and separation of ethyl mercaptan from methane gas on HNb3O8 nanosheets. Ind Eng Chem Res 60:8504–8515. https://doi.org/10.1021/acs.iecr.1c00460
Zhao Y, Chen D, Liu J, He DD, Cao XH, Han CY, Lu JC, Luo YM (2020) Tuning the metal-support interaction on chromium-based catalysts for catalytically eliminate methyl mercaptan: anchored active chromium species through surface hydroxyl groups. Chem. Eng. J. 389:124384. https://doi.org/10.1016/j.cej.2020.124384
Zhu JC, Hu LF, He J, Zhang X, Guo CL (2021) Morphology and surface hydroxyl engineering of H4Nb6O17 nanotubes for enhanced ethyl mercaptan removal. J. Nat. Gas Sci. Eng. 94:104133. https://doi.org/10.1016/j.jngse.2021.104133
Funding
This work was supported by the Anhui Provincial Natural Science Foundation [grant number 2008085QE194], Research Foundation of the Institute of Environment-friendly Materials and Occupational Health of Anhui University of Science and Technology (Wuhu) [grant numbers ALW2020YF01, ALW2020YF09], Foundation of Anhui University of Science and Technology [grant number 13210667] and Innovation Fund Project of Anhui University of Science and Technology Graduate [grant number 2021CX2095].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Lifang Hu and Xin He. The first draft of the manuscript was written by Lifang Hu. Lifang Hu, Jie He, and Jichao Zhu commented on previous versions of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Sami Rtimi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, L., He, X., He, J. et al. Adsorption and photocatalytic conversion of ethyl mercaptan to diethyl disulfide on Fe2O3-loaded HNbMoO6 nanosheet. Environ Sci Pollut Res 29, 75417–75430 (2022). https://doi.org/10.1007/s11356-022-21146-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21146-5