Abstract
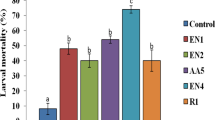
The microbial interactions with plant hosts were known to establish plant growth and beneficial productivity. Some bacteria, fungi, actinomycetes, yeast, and algae have proven as potential effective microbes in agricultural field. In this study, the insecticidal effect of Geobacillus thermodenitrificans PS41 secondary metabolites was tested against third instar larvae of Spodoptera litura, with mortality rate 60.26 ± 1.5% which might influence the agropest management. The test bacterial metabolites were subjected to GC-MS analysis. Totally, 17 different compounds were identified from the ethyl acetate extract metabolites of PS41 strain. The highest peak was obtained with behenic alcohol compound followed by 1-octadecene and penta erythrityl tetrachloride. The Geobacillus thermodenitrificans PS41 secondary metabolites showed potential antifungal activity against plant pathogenic fungi. The highest inhibit was attained against Cladosporium sp., (25 mm) followed by Rhizoctonia solani and Alternaria brassicola (23 mm). However, no toxic effect was exerted upon earthworm (Perionyx excavatus) when treated with PS41 bacterial metabolites. The potential PS41 strain was also found supporting the plant growth. The potential bacterial strain PS41 did not show antagonistic activity against soil bacteria such as Rhizobium sp., Azotobacter sp., Azospirullum brasilense, Pseudomonas fluorescens and Bacillus megaterium. The potential test organism, Geobacillus thermodenitrificans PS41, possessing biopesticide and biofertilizer properties can be a suitable ecofriendly organic applicant in agricultural field for enhancing crop production.




Similar content being viewed by others
Availability of data and materials
Supporting data presented in this study are available on request from the corresponding author
References
Abbasdokht H, Gholami A (2010) The effect of seed inoculation (Pseudomonas putida+ Bacillus lentus) and different levels of fertilizers on yield and yield components of wheat (Triticumaestivum L.) cultivars. World Aced Sci Eng Technol 68:979–983
Adane K, Moore D, Archer SA (1996) Preliminary studies on the use of Beauveria bassiana to control Sitophilus zeamais (Coleoptera:Curculionidae) in the laboratory. J Stored Prod Res 32:105–113
Ajilogba CF, Babalola OO (2019) GC–MS analysis of volatile organic compounds from Bambara groundnut rhizobacteria and their antibacterial properties. World J Microbiol Biotechnol 35(83):1–19
Arfarita N, Hidayati N, Rosyidah A, Machfudz M, Higuchi T (2016) Exploration of indigenous soil bacteria producing-exopolysaccharides for stabilizing of aggregates land potential as biofertilizer. J Degrad Mining Lands Manage 4(1):697–702
Arora NK, Kang SC, Maheshwari DK (2001) Isolation of siderophore-producing strains of Rhizobium melilotiand their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr Sci 81(6):673–674
Asari S, Matzen S, Petersen MA, Bejai S, Meijer J (2016) Multiple effects of Bacillusamylo liquefaciens volatile compounds: plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiol Ecol 92(6):1–11
Beneduzi A, Ambrosini A, Passaglia LM (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35(4):1044–1051
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal biochem 44(1):276–287
Berg G (2009) Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol l84(1):11–18
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195(1):133–140
Cappuccino JG, Sherman N (1992) Biochemical activities of microorganisms. In: Microbiology, a laboratory manual. The Benjamin/Cummings Publishing Co., California
Castric PA (1975) Hydrogen cyanide, a secondary metabolite of Psuedomonas aeruginosa. Can J Microbiol 21:613–618
Chaiharn M, Chunhaleuchanon S, Kozo A, Lumyong S (2005) Screening of rhizobacteria for their plant growth promoting activities. KMITL Sci Technol J 8(1):18–23
Da Costa ACA, Duta FP (2001) Bioaccumulation of copper, zinc, cadmium and lead by Bacillus sp., Bacillus cereus, Bacillussphaericusand Bacillus subtilis. Braz J Microbiol 32:1–5
Devaprasad V, Jayaraj S, Rabindra RJ, Reddy GPV (1990). Studies on the interaction of certain botanicals and nuclear polyhydrosis virus against tobacco caterpillar, Spodoptera litura F. In: Eds, Botanical Pesticide Integrated Pest Management, CTRI, Rajmundry
Dobereiner J (1988) Isolation and identification of root associated diazotrophs. Plant Soil 110:207–212
Gamil WE, Mariy FM, Youssef LA, Abdel Halim SM (2011) Effect of indoxacarb on some biological and biochemical aspects of Spodoptera littoralis (Boisd.) larvae. Ann Agric Sci 56(2):121–126
Gao H, Li P, Xu X, Zeng Q, Guan W (2018) Research on volatile organic compounds from Bacillus subtilis CF-3:biocontrol effects on fruit fungal pathogens and dynamic changes during fermentation. Front Microbiol 9(456):1–15
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol Biochem 37(3):395–412
Gusmiaty, Restu M, Bachtiar B, Larekeng SH (2019) Gibberellin and IAA production by rhizobacteria from various private forest. IOP Conf Series: Earth Environ Sci 270:012018
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Islam MR, Ahamed R, Rahman MO, Akbar MA, Al-Amin M, Alam KD (2010) In vitro antimicrobial activities of four medicinally important plants in Bangladesh. Eur J Sci Res 39:199–206
Jeong JH, Lee OM, Jeon YD, Kim JD, Lee NR (2010) Production of keratinolytic enzyme by a newly isolated feather degrading Stenotrophomonas maltophilia that produces plant growth promoting activity. Process Biochem 45:1738–1745
Jing Y, He Z, Yang X (2007) Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J Zhejiang Univ Sci B 8(3):192–207
Karmarkar R, Pallab P, Dipakranjan M (2014) P hthalides and Phthalans: synthetic methodologies and their applications in the total synthesis. Chem Rev 114(12):6213–6284
Kaur T, Vasudev A, Sohal SK, Manhas RK (2014) Insecticidal and growth inhibitory potential of Streptomyces hydrogenans DH16 on major pest of India, Spodoptera litura (Fab.)(Lepidoptera: Noctuidae). BMC Microbiol 14(1):227
Kennedy JS, Banu BS, Rabindra RJ (2001) Entomopathogenic fungi for the management of diamond block moth Plutella xylostellaon cauliflower. In: Micorbials in insect pest management, (Eds.). Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi
Kloepper JW, Lifshitz R, Zablotowicz RM (1989) Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol 7(2):39–44
Koul O (2011) Microbial biopesticides: opportunities and challenges. CAB Rev: PerspesctivesAgriVeterSciNutr Nat Res 6(056):1–26
Koul O, Singh G, Singh R, Multani JS (2005) Bioefficacy and mode of action of Aglaroxin B and Aglaroxin C from Aglaiae laeagnoidea (syn. A. roxburghiana) against Helicoverpaarmigera and Spodopteralitura. Entomol Exp et Applicata 114:197–204
Kshetri P, Ningthoujam DS (2016) Keratinolytic activities of alkaliphilicBacillus sp. MBRL 575 from a novel habitat, limestone deposit site in Manipur, India. SpringerPlus 5(595):1–16
Kumar A, Kumar A, Devi S, Patil S, Negi CPS (2012) Isolation, screening and characterization of bacteria from rhizospheric soils for different plant growth promotion (PGP) activities: an in vitro study. Recent Res Sci Technol 4(1):01–05
Li XY, Mao ZC, Wu YX, Ho HH, He YQ (2015) Comprehensive volatile organic compounds profiling of Bacillus species with biocontrol properties by head space solid phase microextraction with gas chromatography-mass spectrometry. Biocont Sci Technol l25:132–143
Liu Y, Zhou Q, Xie X, Lin D, Dong L (2010) Oxidative stress and DNA damage in the earthworm Eiseniafetida induced by toluene, ethylbenzene and xylene. Ecotoxicol 19(8):1551–1559
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Lugtenberg BJ, Dekkers L, Bloemberg GV (2001) Molecular determinants of rhizosphere colonization by Pseudomonas. Annu Rev Phytopathol 39(1):461–490
Majeed A, Abbasi MK, Hameed S, Imran A, Rahim N (2015) Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front Microbiol 6:198
Manjula K, Krishna Murthy KV (2005) Efficacy of Nomuraearileyi against different instars of Spodoptera litura and Helico verpaarmigera. Ann Plant Protect Sci 13(2):347–350
Namasivayam SKR, Sekar S, Arvind Bharani RS (2014) Pesticidal activity of endophytic fungal metabolites against major groundnut defoliator Spodoptera litura (Fab.) (Lepidoptera:Noctuidae). J Biopest 7:116–121
Neeraja C, Anil K, Purushotham P, Suma K, Sarma PV, Moerschbacher BM, Podile AR (2010) Biotechnological approaches to develop bacterial chitinases as a bioshield against fungal diseases of plants. Crit Rev Biotechnol 30(3):231–241
Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68(8):3795–3801
Phugare SS, Gaikwad YB, Jadhav JP (2012) Biodegradation of acephate using a developed bacterial consortium and toxicological analysis using earthworms (Lumbricusterrestris) as a model animal. Int Biod etBiode grad 69:1–9
Poonguzhali S, Madhaiyan M, Sa TM (2008) Isolation and identification of phosphate solubilizing bacteria from Chinese cabbage and their effect on growth and phosphorus utilization of plants. J Microbiol Biotechnol 18:773–777
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Loccoz YM (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Raguvaran K, Kalpana M, Manimegalai T, Maheswaran R (2021) Insecticidal, not-target organism activity of synthesized silver nanoparticles using Actinokineospora fastidiosa. Biocat Agricul Biotechnol 38:102197
Rajawat MV, Singh S, Tyagi SP, Saxena AK (2016) A modified plate assay for rapid screening of potassium-solubilizing bacteria. Pedosphere 26(5):768–773
Rajer FU, Wu H, Xie Y, Xie S, Raza W, Tahir HAS, Gao X (2017) Volatile organic compounds produced by a soil isolate, Bacillus subtilis FA26 induce adverse ultra-structural changes to the cells of Clavibactermichiganensisssp. sepedonicus, the causal agent of bacterial ring rot of potato. Microbiology 163:523–530
Roh JY, Choi JY, Li MS, Jin BR, Je YH (2007) Bacillusthuringiensis as a specific, safe, and effective tool for insect pest control. J Microbiol Biotechnol 17(4):547
Sagahon IP, Anducho-Reyes MA, Silva-Rojas HV, Arana-Cuenca A, Tellez-Jurado A, Cardenas-Álvarez IO, Mercado-Flores Y (2011) Isolation of bacteria with antifungal activity against the phytopathogenic fungi Stenocarpella maydis and Stenocarpella macrospora. Int J Mol Sci 12(9):5522–5537
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 21(1):1–30
Saravanan VS, Subramoniam SR, Raj SA (2003) Assessing in vitro solubilization potential of different zinc solubilizing bacterial (zsb) isolates. Braz J Microbiol 34:121–125
Sarita N, Mohari MP, Ghodki BS, Lande GK, Bisane KD, Thakare AS, Barkhade UP (2010) Biochemical analysis and synergistic suppression of indoxacarb resistance in Plutella xylostella L. J Asia Pac Entomol 13(2):91–95
Shahid M, Hameed S, Imran A, Ali S, Van Elsas JD (2012) Root colonization and growth promotion of sunflower (Helianthus annuus L.) by phosphate solubilizing Enterobacter sp. Fs-11. World J Microbiol Biotechnol 28:2749–2758
Sivasakthivelan P, Saranraj P (2013) Azospirillum and its formulations: a review. Int J Microbiol Res 4(3):275–282
Skariyachan S, Rao AG, Patil MR, Saikia B, BharadwajKn V, RaoGs J (2013) Antimicrobial potential of metabolites extracted from bacterial symbionts associated with marine sponges in coastal area of Gulf of Mannar Biosphere. India Let Appl Microbiol 58:231–241
Soylu S, Soylu EM, Kurt S, Ekici OK (2005) Antagonistic potentials of rhizosphere-associated bacterial isolates against soil-borne diseases of tomato and pepper caused by Sclerotinia sclerotiorum and Rhizoctonia solani. Pak J Biol Sci 8:43–48
Stefan M, Mihasan M, Dunca S (2008) Plant growth promoting rhizobacteria can inhibit the in vitro germination of Glycine max L. seeds. J Exp Mol Bio 9(3)
Tabashnik BE, Van Rensburg JB, Carriere Y (2009) Field-evolved insect resistance to Bt crops: definition, theory, and data. J Econ Entomol 102(6):2011–2025
Tariq M, Yasmin S, Hafeez FY (2010) Biological control of potato black scurf by rhizosphere associated bacteria. Braz J Microbiol 41(2):439–451
Wadyalkar LSR, Buldo AN (2002) Effect of phosphate solubilizing bacteria on tricalium phosphate containing media. In: Proc. 15th International Meeting on Microbial Phosphate Solubilization. Salamanaca University, Salamanca, pp 16–19
Williams GE, Asher MJ (1996) Selection of rhizobacteria for the control of Pythium ultimum and Aphanomycescochlioides on sugar-beet seedlings. Crop Prot 15(5):479–486
Yuan J, Raza W, Shen Q, Huang Q (2012) Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl Environ Microbiol 78:5942–5944
Yuvaraj A, Karmegam N, Thangaraj R (2018) Vermistabilization of paper mill sludge by an epigeic earthworm Perionyx excavatus: mitigation strategies for sustainable environmental management. Ecol Eng 120:187–197
Zhang X, Li B, Wang Y, Guo Q, Lu X, Li S, Ma P (2013) Lipopeptides, a novel protein, and volatile compounds contribute to the antifungal activity of the biocontrol agent Bacillus atrophaeus CAB-1. Appl Microbiol Biotechnol 97:9525–9534
Acknowledgements
DST-FIST, New Delhi, India, granted sophisticated instrumentation facility with reference number SR/ FST/ LSI-640/ 2015 (C) dated 30/5/2016 and financial support under DBT (BT/PR 5426/AAQ/3/599/2012) New Delhi, Virbac Animal Health India Pvt. Ltd. (VIRBAC - Consultancy project sanction order, Date: 30.10.2018), Mumbai, in the form of fellowship.
Author information
Authors and Affiliations
Contributions
Dr. Hemalatha N (Corresponding author) — research idea, work plan and paper work
Dr. Siddharthan N (First author) — implementation and result data collection, manuscript typing
Dr. Balagurunathan R — chemical and instrumental facilities, paper editing
Dr. Venkatesan S — data analysis
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
We confirm that if the paper is accepted, it could be published in this prestigious journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siddharthan, N., Balagurunathan, R., Venkatesan, S. et al. Bio-efficacy of Geobacillus thermodenitrificans PS41 against larvicidal, fungicidal, and plant growth–promoting activities. Environ Sci Pollut Res 30, 42596–42607 (2023). https://doi.org/10.1007/s11356-022-20455-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20455-z