Abstract
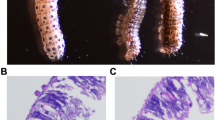
The fall armyworm, Spodoptera frugiperda, has become a worldwide pest and threatens world food production. A previous study indicated that azadirachtin, the most effective botanical insecticide for S. frugiperda, inhibits larval growth of the insect. The effect of azadirachtin on the tissues of the larvae, however, remains to be determined. In this study, the effects of azadirachtin on the structure of fat bodies were analyzed. Comparative transcriptomic analysis was conducted between controls and samples treated with 0.1 μg/g azadirachtin for 7 days to explore potential relevant mechanisms. The expression of 5356 genes was significantly affected after azadirachtin treatment, with 3020 up-regulated and 2336 down-regulated. Among them, 137 encode detoxification enzymes, including 53 P450s, 20 GSTs, 27 CarEs, 16 UGTs, and 12 ABC transporters. Our results indicated that azadirachtin could destroy fat body structure and change the mRNA levels of detoxification-related genes. The up-regulated genes encoding detoxification enzymes might be related to detoxifying azadirachtin. Our results elucidate a preliminary mechanism of azadirachtin detoxification in the fat bodies of S. frugiperda larvae.



Similar content being viewed by others

Data availability
The raw reads of transcriptomes in this study have been deposited in the NCBI SRA database with the accession number from PRJNA686499.
References
Adedipe F, Grubbs N, Coates B, Wiegmman B, Lorenzen M (2019) Structural and functional insights into the Diabrotica virgifera virgifera ATP-binding cassette transporter gene family. BMC Genomics 20(1):899
Ahmad S, Ansari MS, Moraiet MA (2013) Demographic changes in Helicoverpa armigera after exposure to Neemazal (1% EC azadirachtin). Crop Prot 50:30–36
Ahn SJ, Vogel H, Heckel DG (2012) Comparative analysis of the UDP-glycosyltransferase multigene family in insects. Insect Biochem Mol Biol 42(2):133–147
Carvalho RA, Omoto C, Field LM, Williamson MS, Bass C (2013) Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PLoS One 8(4):e62268
Chen X, Palli SR (2021) Transgenic overexpression of P450 genes confers deltamethrin resistance in the fall armyworm, Spodoptera frugiperda. J Pest Scihttps://doi.org/10.1007/s10340-021-01452-6
Enayati AA, Ranson H, Hemingway J (2005) Insect glutathione transferases and insecticide resistance. Insect Mol Biol 14:3–8
Feyereisen R (1999) Insect P450 enzymes. Annu Rev Entomol 44:507–533
Firake DM, Behere GT (2020) Bioecological attributes and physiological indices of invasive fall armyworm, Spodoptera frugiperda (J. E. Smith) infesting ginger (Zingiber officinale Roscoe) plants in India. Crop Protect 137:105233
Genta FA, Souza RS, Garcia ES, Azambuja P (2010) Phenol oxidases from Rhodnius prolixus: temporal and tissue expression pattern and regulation by ecdysone. J Insect Physiol 56(9):1253–1259
Goergen G, Kumar PL, Sankung SB, Togola A, Tamò M (2016) First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS One 11(10):e0165632
Greenberg SM, Showler AT, Liu TX (2005) Effects of neem-based insecticides on beet armyworm (Lepidoptera: Noctuidae). Insect Sci 12:17–23
Guedes CA, Teixeira VW, Dutra KA, Navarro D, Cruz GS, Lapa Neto C, Correia AA, Sandes JM, Brayner FA, Alves LC, Teixeira Á (2020) Evaluation of Piper marginatum (Piperales: Piperaceae) oil and geraniol on the embryonic development of Spodoptera frugiperda (Lepidoptera: Noctuidae) in comparison to formulated products. J Econ Entomol 113(1):239–248
Gui F, Lan T, Zhao Y, Guo W, Dong Y, Fang D, Liu H, Li H, Wang H, Hao R, Cheng X, Li Y, Yang P, Sahu SK, Chen Y, Cheng L, He S, Liu P, Fan G, Lu H, Hu G, Dong W, Chen B, Jiang Y, Zhang Y, Xu H, Lin F, Slipper B, Postma A, Jackson M, Abate BA, Tesfaye K, Demie AL, Bayeleygne MD, Degefu DT, Chen F, Kuria PK, Kinyua ZM, Liu T, Yang H, Huang F, Liu X, Sheng J, Kang L (2020) Genomic and transcriptomic analysis unveils population evolution and development of pesticide resistance in fall armyworm Spodoptera frugiperda. Protein Cell 20(6):1682–1696
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hafeez M, Li X, Zhang Z, Huang J, Wang L, Zhang J, Shah S, Khan MM, Xu F, Fernández-Grandon GM, Zalucki MP, Lu Y (2021) De novo transcriptomic analyses revealed some detoxification genes and related pathways responsive to noposion Yihaogong® 5% EC (lambda-cyhalothrin 5%) exposure in Spodoptera frugiperda third-instar larvae. Insects 12(2):132
Hemingway J, Hawkes NJ, McCarroll L, Ranson H (2004) The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol 34(7):653–665
Hu B, Hu S, Huang H, Wei Q, Ren M, Huang S, Tian X, Su J (2019) Insecticides induce the co-expression of glutathione S-transferases through ROS/CncC pathway in Spodoptera exigua. Pestic Biochem Phys 155:58–71
Hummel H, Langner S, Leithold G, Schmutterer H (2014) Neem: unusually versatile plant genus azadirachta with many useful and so far insufficiently exploited properties for agriculture, medicine, and industry. Commun Agric Appl Biol Sci 79:211–228
Isman MB, Koul O, Luczynski A, Kaminski J (1990) Insecticidal and antifeedant bioactivities of neem oils and their relationship to azadirachtin content. J Agric Food Chem 38:1406–1411
Isman MB (2006) Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51:45–66
Jing DP, Guo JF, Jiang YY, Zhao JZ, Sethi A, He KL, Wang ZY (2019) Initial detections and spread of invasive Spodoptera frugiperda in China and comparisons with other noctuid larvae in cornfields using molecular techniques. Insect Sci 27(4):1–11
Josephrajkumar A, Subrahmanyam B, Srinivasan (1999) Plumbagin and azadirachtin deplete hemolymph ecdysteroid levels and alter the activity profiles of two lysosomal enzymes in the fat body of Helicoverpa armigera (Lepidoptera: Noctuidae). Eur J Entomol 96(4):347–353
Li JJ, Jin MH, Wang NM, Yu QT, Shang ZY, Xue CB (2020) Combined transcriptomic and proteomic analysis of flubendiamide resistance in Plutella xylostella. Entomol Res 50(10):483–492
Li S, Yu X, Feng Q (2019) Fat body biology in the last decade. Annu Rev Entomol 64:315–333
Li YQ, Bai LS, Zhao CX, Xu JJ, Sun ZJ, Dong YL, Li DX, Liu XL, Ma ZQ (2020) Functional characterization of two carboxylesterase genes involved in pyrethroid detoxification in Helicoverpa armigera. J Agric Food Chem 68(11):3390–3402
Li X, Schuler MA, Berenbaum MR (2007) Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol 52:231–253
Li X, Shi H, Gao X, Liang P (2018) Characterization of UDP-glucuronosyltransferase genes and their possible roles in multi-insecticide resistance in Plutella xylostella (L.). Pest Manag Sci 74(3):695–704
Lu K, Song Y, Zeng R (2021) The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr Opin Insect Sci 43:103–107
Mackenzie PI, Owens IS, Burchell B, Bock KW, Bairoch A, Bélanger A, Fournel-Gigleux S, Green M, Hum DW, Iyanagi T, Lancet D, Louisot P, Magdalou J, Chowdhury JR, Ritter JK, Schachter H, Tephly TR, Tipton KF, Nebert DW (1997) The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 7(4):255–269
Mao T, Li F, Fang Y, Wang H, Chen J, Li M, Lu Z, Qu J, Li J, Hu J, Cheng X, Ni M, Li B (2019) Effects of chlorantraniliprole exposure on detoxification enzyme activities and detoxification-related gene expression in the fat body of the silkworm, Bombyx mori. Ecotoxicol Environ Saf 176:58–63
McComic SE, Rault LC, Anderson TD, Swale DR (2020) Reduced neuronal sensitivity and susceptibility of the fall armyworm, Spodoptera frugiperda, to pyrethroids in the absence of known knockdown mutations. Pestic Biochem Phys 169:104652
Meng X, Yang X, Wu Z, Shen Q, Miao L, Zheng Y, Qian K, Wang J (2020) Identification and transcriptional response of ATP-binding cassette transporters to chlorantraniliprole in the rice striped stem borer. Chilo Suppressalis Pest Manag Sci 76(11):3626–3635
Montezano DG, Specht A, Sosa-Gómez DR, Roque-Specht VF, Sousa-Silva JC, Paula-Moraes SV, Peterson JA, Hunt TE (2018) Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr Entomol 26:286–300
Mordue AJ, Blackwell A (1993) Azadirachtin: an update. J Insect Physiol 39:903–924
Muraro DS, Neto DDA, Kanno RH, Kaiser IS, Bernardi O, Omoto C (2021) Inheritance patterns, cross-resistance and synergism in Spodoptera frugiperda (Lepidoptera Noctuidae) resistant to emamectin benzoate. 77(11): 5049–5057
Nauen R, Zimmer CT, Vontas J (2020) Heterologous expression of insect P450 enzymes that metabolize xenobiotics. Cur Opin Insect Sci 43:78–84
Oulhaci CM, Denis B, Kilani-Morakchi S, Sandoz JC, Kaiser L, Joly D, Aribi N (2018) Azadirachtin effects on mating success, gametic abnormalities and progeny survival in Drosophila melanogaster (Diptera). Pest Manag Sci 74(1):174–180
Padovez FEO, Kanno RH, Omoto C, Guidolin AS (2021) Fitness costs associated with chlorantraniliprole resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) strains with different genetic backgrounds. Pest Manag Sci. https://doi.org/10.1002/ps.6746
Pan Y, Wen S, Chen X, Gao X, Zeng X, Liu X, Tian F, Shang Q (2020) UDP-glycosyltransferases contribute to spirotetramat resistance in Aphis gossypii Glover. Pestic Biochem Physiol 166:104565
Scudeler EL, Garcia A, Padovani CR, Dos Santos DC (2019) Pest and natural enemy: how the fat bodies of both the southern armyworm Spodoptera eridania and the predator Ceraeochrysa claveri react to azadirachtin exposure. Protoplasma 256(3):839–856
Schmutterer H (1990) Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Ann Rev Entomol 35:271–297
Shu B, Yu H, Li Y, Zhong H, Li X, Cao L, Lin J (2021) Identification of azadirachtin responsive genes in Spodoptera frugiperda larvae based on RNA-seq. Pestic Biochem Phys 172:104745
Shu B, Zou Y, Yu H, Zhang W, Li X, Cao L, Lin J (2021) Growth inhibition of Spodoptera frugiperda larvae by camptothecin correlates with alteration of the structures and gene expression profiles of the midgut. BMC Genomics 22:391
Sparks AN (1979) A review of the biology of the fall armyworm. Fla Entomol 62:82–87
Sturm A, Cunningham P, Dean M (2009) The ABC transporter gene family of Daphnia pulex. BMC Genomics 10:170
Tambo JA, Kansiime MK, Mugambi I, Rwomushana I, Kenis M, Day RK, Lamontagne-Godwin J (2020) Understanding smallholders’ responses to fall armyworm (Spodoptera frugiperda) invasion: evidence from five African countries. Sci Total Environ 740:140015
Tang B, Dai W, Qi L, Du S, Zhang C (2020) Functional characterization of an α-esterase gene associated with malathion detoxification in Bradysia odoriphaga. J Agric Food Chem 68(22):6076–6083
Tojo S, Kiguchi K, Kimura S (1981) Hormonal control of storage protein synthesis and uptake by the fat body in the silkworm. Bombyx Mori J Insect Physiol 27(7):491–497
Ullah F, Gul H, Tariq K, Desneux N, Gao X, Song DL (2020) Functional analysis of cytochrome P450 genes linked with acetamiprid resistance in melon aphid. Aphis gossypii. Pestic Biochem Phys 170:104687
Wheelock CE, Shan G, Ottea J (2005) Overview of carboxylesterases and their role in the metabolism of insecticides. J Pestic Sci 30:75–83
Xu L, Mei Y, Liu R, Chen X, Li D, Wang C (2020) Transcriptome analysis of Spodoptera litura reveals the molecular mechanism to pyrethroids resistance. Pestic Biochem Physiol 169:104649
Yu SJ (1991) Insecticide resistance in the fall armyworm, Spodoptera-frugiperda (Smith, J. E.). Pestic Biochem Physiol 39(1):84–91
Yu SJ, Nguyen SN, Abo-Elghar GE (2003) Biochemical characteristics of insecticide resistance in the fall armyworm, Spodoptera frugiperda (J.E. Smith). Pestic Biochem Physiol 77:1–11
Zhang D, Xiao Y, Xu P, Yang X, Wu Q, Wu K (2021) Insecticide resistance monitoring for the invasive populations of fall armyworm, Spodoptera frugiperda in China. J Integr Agr 20(3):783–791
Acknowledgements
The authors sincerely thank Prof. Ming-Shun Chen of Kansas State University for revising this manuscript.
Funding
This work was financially supported by the fund from Key-Area Research and Development Program of Guangdong Province (no. 2020B020223004), the National Natural Science Foundation of China (grant no. 32102221), the Innovation Team Project in Guangdong Provincial Department of Education (2017KCXTD018), and Guangzhou Science and Technology Plan Projects (grants 201803020009 and 201903010043).
Author information
Authors and Affiliations
Contributions
HY, XY, JD, and BS performed the experiments, and YL and SV helped with the data analysis. BS drafted the manuscript. BS and JL conceived of the study, participated in its design and coordination, and helped to revise the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Giovanni Benelli.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, H., Yang, X., Dai, J. et al. Effects of azadirachtin on detoxification-related gene expression in the fat bodies of the fall armyworm, Spodoptera frugiperda. Environ Sci Pollut Res 30, 42587–42595 (2023). https://doi.org/10.1007/s11356-022-19661-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19661-6