Abstract
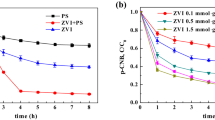
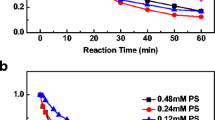
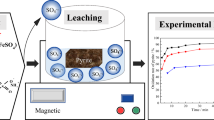
This study revealed a dual pathway for the degradation of tris(1-chloro-2-propanyl) phosphate (TCPP) by zero-valent iron (ZVI) and persulfate as co-milling agents in a mechanochemical (MC) process. Persulfate was activated with ZVI to degrade TCPP in a planetary ball mill. After milling for 2 h, 96.5% of the TCPP was degraded with the release of 63.16, 50.39, and 42.01% of the Cl−, SO42−, and PO43−, respectively. In the first degradation pathway, persulfate was activated with ZVI to produce hydroxyl (·OH) radicals, and ZVI is oxidized to Fe(II) and Fe(III). A substitution reaction occurred as a result of the attack of ·OH on the P–O–C bonds, leading to the successive breakage of the three P–O–C bonds in TCPP to produce PO43−. In the second pathway, a C–Cl bond in part of the TCPP molecule was oxidized by SO4·− to carbonyl and carboxyl groups. The P–O–C bonds continued to react with ·OH to produce PO43−. Finally, the intermediate organochloride products were further reductively dechlorinated by ZVI. However, the synergistic effect of the oxidation (·OH and SO4·−) and the reduction reaction (ZVI) did not completely degrade TCPP to CO2, resulting in a low mineralization rate (35.87%). Moreover, the intermediate products still showed the toxicities in LD50 and developmental toxicant. In addition, the method was applied for the degradation of TCPP in soil, and high degradations (> 83.83%) were achieved in different types of soils.







Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdellaoui M, Gaffet E (1995) The physics of mechanical alloying in a planetary ball mill: Mathematical treatment. Acta Metall Mater 43:339–344
Al-Shamsi MA, Thomson NR (2013) Treatment of organic compounds by activated persulfate using nanoscale zerovalent iron. Ind Eng Chem Res 52:13564–13571
Björklund J, Isetun S, Nilsson U (2004) Selective determination of organophosphate flame retardants and plasticizers in indoor air by gas chromatography, positive-ion chemical ionization and collision-induced dissociation mass spectrometry. Department of Analytical Chemistry, Stockholm University, S-106 91 Stockholm. Sweden 18:3079–3083
Cagnetta G, Robertson J, Huang J, Zhang K, Yu G (2016) Mechanochemical destruction of halogenated organic pollutants: a critical review. J Hazard Mater 313:85–102
Cagnetta G, Huang J, Lu M, Wang B, Wang Y, Deng S, Yu G (2017) Defect engineered oxides for enhanced mechanochemical destruction of halogenated organic pollutants. Chemosphere 184:879–883
Cheng Z, Chen Q, Cervantes S, Tang Q, Gao X, Tan Y, Liu S, Ma Y, Shen Z (2020) Two-dimensional and three-dimensional quantitative structure-activity relationship models for the degradation of organophosphate flame retardants during supercritical water oxidation. J Hazard Mater 394:121811
Deng S, Bao Y, Cagnetta G, Huang J, Yu G (2020) Mechanochemical degradation of perfluorohexane sulfonate: synergistic effect of ferrate(VI) and zero-valent iron. Environ Pollut 264:114789
Dong Y, Li Y, Zhao C, Feng Y, Dong Y (2019) Mechanism of the rapid mechanochemical degradation of hexachlorobenzene with silicon carbide as an additive. J Hazard Mater 379:120653
Dubinskaya AM (2010) Transformations of organic compounds under the action of mechanical stress. Russ Chem Rev 68:637–652
Fan G, Liu X, Lin C, Li PJEC (2018) Peroxymonosulfate assisted mechanochemical method for the degradation of phenanthrene in contaminated soils. Emerg Contam 4:22–31
Fan G, Liu X, Li X, Lin C, He M, Ouyang W (2020) Mechanochemical treatment with CaO-activated PDS of HCB contaminated soils. Chemosphere 257:127207
Hoffman K, Daniels JL, Stapleton HM (2014) Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int 63:169–172
Jurgens SS, Helmus R, Waaijers SL, Uittenbogaard D, Dunnebier D, Vleugel M, Kraak MHS, De Voogt P, Parsons JR (2014) Mineralisation and primary biodegradation of aromatic organophosphorus flame retardants in activated sludge. Chemosphere 111:238–242
Kishi K, Ikeda S (1973) X-Ray photoelectron spectroscopic study for the reaction of evaporated iron with O and H2O. Bull Chem Soc Jpn 46:341–345
Li D, Zhong Y, Zhu X, Wang H, Yang W, Deng Y, Huang W, Peng Pa (2021) Reductive degradation of chlorinated organophosphate esters by nanoscale zerovalent iron/cetyltrimethylammonium bromide composites: reactivity, mechanism and new pathways. Water Res 188:116447
Liu X, Zhang X, Shao K, Lin C (2016a) Fe0-activated persulfate-assisted mechanochemical destruction of expired compound sulfamethoxazole tablets. RSC Adv 6:20938–20948
Liu X, Zhang X, Zhang K, Qi C (2016b) Sodium persulfate-assisted mechanochemical degradation of tetrabromobisphenol A: efficacy, products and pathway. Chemosphere 150:551–558
Liu J, Li C, Zhou P, Wu S, Ou H (2017) Heterogeneous photocatalysis of tris(2-chloroethyl) phosphate by UV/TiO2: degradation products and impacts on bacterial proteome. Water Res 124:29–38
Moulder JF, Chastain J, King RC (1992) Handbook of x-ray photoelectron spectroscopy : a reference book of standard spectra for identification and interpretation of XPS data. Chem Phys Lett 220:7–10
Oh SY, Kim HW, Park JM, Park HS, Yoon C (2009) Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J Hazard Mater 168:346–351
Quintana JB, Rodil R, Reemtsma T, García-López M, Rodríguez I (2008) Organophosphorus flame retardants and plasticizers in water and air II. Analytical methodology. TrAC, Trends Anal Chem 27:904–915
Ruan XC, Ai R, Jin X, Zeng QF, Yang ZY (2013) Photodegradation of tri (2-chloroethyl) phosphate in aqueous solution by UV/H2O2. Water Air Soil Pollut 224:1–10
Siow KS, Britcher L, Kumar S, Griesser HJ, Polymers (2014) Deposition and XPS and FTIR analysis of plasma polymer coatings containing phosphorus. Plasma Processes Polym 11:133–141
Sui H, Rong Y, Song J, Zhang D, Li H, Wu P, Shen Y, Huang Y (2017) Mechanochemical destruction of DDTs with Fe-Zn bimetal in a high-energy planetary ball mill. J Hazard Mater 342:201–209
Un-Jung K, Jung K, OhKannan K (2017) Occurrence, removal, and environmental emission of organophosphate flame retardants/plasticizers in a wastewater treatment plant in New York state. Environ Sci Technol 14:7872–7880
Veen IVD, Boer JD (2012) Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88:1119–1153
Wakayama H, Mizuno J, Fukushima Y, Nagano K, Fukunaga T, Mizutani UJC (1999) Structural defects in mechanically ground graphite. Carbon 37:947–952
Wang G, Chen H, Du Z, Li J, Wang Z (2017) In vivo metabolism of organophosphate flame retardants and distribution of their main metabolites in adult zebrafish. Sci Total Environ 590–591:50–59
Wang N, Lv H, Zhou Y, Zhu L, Tang H (2019) Complete defluorination and mineralization of perfluorooctanoic acid by a mechanochemical method using alumina and persulfate. Environ Sci Technol 53:8302–8313
Wang R, Zhu Z, Tan S, Guo J, Xu Z (2020) Mechanochemical degradation of brominated flame retardants in waste printed circuit boards by ball milling. J Hazard Mater 385:121509
Wei X, Gao N, Li C, Yang D, Zhou S, Lei L (2016) Zero-valent iron (ZVI) activation of persulfate (PS) for oxidation of bentazon in water. Chem Eng J 285:660–670
Xu W, Mao N, Zhang J (2013) Graphene: a platform for surface-enhanced raman spectroscopy. Small 9:1206–1224
Yan X, Liu X, Qi C, Wang D, Lin C (2015) Mechanochemical destruction of a chlorinated polyfluorinated ether sulfonate (F-53B, a PFOS alternative) assisted by sodium persulfate. RSC Adv 5:85785–85790
Yan X, Liu X, Qi C, Lin C, Li P, Wang H (2017) Disposal of hexabromocyclododecane (HBCD) by grinding assisted with sodium persulfate. RSC Adv 7:23313–23318
Yin Z, Han M, Hu Z, Feng L, Liu Y, Du Z, Zhang L (2020) Peroxymonosulfate enhancing visible light photocatalytic degradation of bezafibrate by Pd/g-C3N4 catalysts: the role of sulfate radicals and hydroxyl radicals. Chem Eng J 390:124532
Yu X, Yin H, Peng H, Lu G, Dang Z (2019) Degradation mechanism, intermediates and toxicology assessment of tris-(2-chloroisopropyl) phosphate using ultraviolet activated hydrogen peroxide. Chemosphere 241:124991
Zhang W, Huang J, Xu F, Deng S, Zhu W, Yu G (2011) Mechanochemical destruction of pentachloronitrobenzene with reactive iron powder. J Hazard Mater 198:275–281
Zhang Y, Tao H, Li J, Yang X (2020) Achieving a high-performance P/C anode through P-O-C bond for sodium ion batteries. Ionics 26:3377–3385
Funding
This work was financially supported by the Natural Science Foundation of Jiangsu Province (BK20201388), the Industry Prospect and Common Key Technologies in Jiangsu Province (BE2018015), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
W. Qiao and Q. Yang designed the work, performed MC degradation and sampling, and performed all parameters’ measurements used in this project. Y. Qian and Z. Zhang analyzed data and participated in the interpretation of data. W. Qiao and Q. Yang contributed to drafting and critically revising of the paper. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Speciality: Phosphorus flame retardants; Advanced oxidation process; Ball milling
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qiao, W., Yang, Q., Qian, Y. et al. Degradation of tris(1-chloro-2-propanyl) phosphate by the synergistic effect of persulfate and zero-valent iron during a mechanochemical process. Environ Sci Pollut Res 29, 34349–34359 (2022). https://doi.org/10.1007/s11356-022-18665-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18665-6