Abstract
Livers of caribou and reindeer (Rangifer tarandus) from Canada (n = 146), Greenland (n = 30), Svalbard (n = 7), and Sweden (n = 60) were analyzed for concentrations of eight perfluoroalkyl carboxylic acids and four perfluoroalkane sulfonic acids. In Canadian caribou, PFNA (range < 0.01–7.4 ng/g wet weight, ww) and PFUnDA (<0.01–5.6 ng/g ww) dominated, whereas PFOS predominated in samples from South Greenland, Svalbard, and Sweden, although the highest concentrations were found in caribou from Southwest Greenland (up to 28 ng/g ww). We found the highest median concentrations of all PFAS except PFHxS in Akia-Maniitsoq caribou (Southwest Greenland, PFOS 7.2–19 ng/g ww, median 15 ng/g ww). The highest concentrations of ΣPFAS were also found in Akia-Maniitoq caribou (101 ng/g ww) followed by the nearby Kangerlussuaq caribou (45 ng/g ww), where the largest airport in Greenland is situated, along with a former military base. Decreasing trends in concentrations were seen for PFOS in the one Canadian and three Swedish populations. Furthermore, PFNA, PFDA, PFUnDA, PFDoDA, and PFTrDA showed decreasing trends in Canada’s Porcupine caribou between 2005 and 2016. In Sweden, PFHxS increased in the reindeer from Norrbotten between 2003 and 2011. The reindeer from Västerbotten had higher concentrations of PFNA and lower concentrations of PFHxS in 2010 compared to 2002. Finally, we observed higher concentrations in 2010 compared to 2002 (albeit statistically insignificant) for PFHxS in Jämtland, while PFNA, PFDA, PFUnDA, PFDoDA, and PFTrDA showed no difference at all.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Per- and polyfluoroalkyl substances (PFAS) is the generic name for over 4500 chemicals including perfluoroalkyl acids (PFAAs) and their precursor compounds (OECD 2018), including perfluorinated carboxylic acids (PFCAs) and perfluorinated sulfonic acids (PFSAs), which are the most commonly analyzed PFAS. They are fluorinated, man-made chemicals with wide industrial and commercial applications (Kissa 2001). Being both oil and water repellent, PFAS are or have been used in a large number of different applications for over six decades, including in firefighting foams, grease-repellent food wrappers, textile stain and soil repellents, cleaning aids, processing aids in fluoropolymer manufacturing, ski wax, nonstick cookware, outdoor weather-repellent textiles, carpets and leather, etc. (Kotthoff et al. 2015; Prevedouros et al. 2006; Wang et al. 2013, 2015). They are tenaciously persistent in the environment, found in several abiotic and biotic remote Arctic samples (Muir et al. 2019), and released into the environment either directly or indirectly through their life cycle from industrial production to their usage in consumer products. Several episodes of direct release of PFOS into the environment because of firefighting activities have been documented (Li et al. 2018; Moody et al. 2002). Meanwhile, PFOA was used as a processing aid in the manufacturing of tetrafluoroethylene-based fluoropolymers. PFOA is also a degradation product of fluorotelomer precursors including fluorotelomer alcohols (FTOHs) which are used to make polyfluoro-based products.
The largest producer of PFOS and PFOS-based compounds, the 3M Company, voluntarily phased out their production of PFOS in 2001 after evidence of elevated PFOS concentrations in blood from employees as well as in wildlife (3M Phase-out plan for POSF-based products, 2000; Giesy and Kannan 2001), and the European Union restricted the use of PFOS in 2006 (EC 2006). From the mid-2000s, production has increased in China (Xie et al. 2013). In 2009, PFOS, its salts, and perfluorooctane sulfonyl fluoride (PFOS-F, which degrade to PFOS in the environment) were added under Annex B of the Stockholm Convention of Persistent Organic Pollutants (POPs) (http://www.pops.int/) and PFOA was later added to Annex A.
In terms of toxicity of PFAS, PFOS is the most investigated PFSA, while PFOA is the most investigated PFCA. The highest concentrations of PFOS and PFOA in humans and wildlife are usually found in the liver, kidney, and blood (Butt et al. 2010; Houde et al. 2011). PFOS has a negative effect on the immune system (Budtz-Jørgensen and Grandjean 2018; De Witt et al. 2012; Petersen et al. 2018). Furthermore, PFOS and PFOA have been shown to cause increased liver weight and hepatocytic hypertrophy (Kennedy et al. 2004; Seacat et al. 2003), abnormal behavior, weight loss, and serious damage in liver and lung tissue (Cui et al. 2008) as well as developmental neurotoxic effects (Johansson et al. 2008).
PFAS occurrence in humans and wildlife is global (Butt et al. 2010); they are found in the Antarctic environment (Schiavone et al. 2009) as well as the Arctic (Muir et al. 2019). Most wildlife studies have focused on the aquatic food chain, where the highest concentrations usually are found. Studies of PFAS in Arctic terrestrial animals are relatively few (Aas et al. 2014; Bossi et al. 2015; Butt et al. 2010; Larter et al. 2017; Martin et al. 2004; Muir et al. 2019; Müller et al. 2011; Routti et al. 2017).
Caribou and reindeer are the same species, Rangifer tarandus, but there are several subspecies. They are an important food resource for people throughout the Arctic and an intrinsic component of traditional culture and food for many northern peoples. PFOS and perfluorinated carboxylates (PFCA C7–C11) were measured in traditional foods collected in Nunavut, Canada, between 1997 and 1999. Highest concentrations of total PFAS were found in caribou liver and the authors concluded that caribou meat contributed 43–75% of daily PFAS dietary exposure in the area (Ostertag et al. 2009). With their circumpolar distribution, caribou and reindeer are a suitable species for investigations of spatial trends across the Arctic. The main transport route for PFAS, which are directly released into aquatic systems, are ocean currents, whereas volatile precursors of PFSA and PFCAs undergo long-range atmospheric transport and degrade to PFSA and PFCAs in the atmosphere and cryosphere (see Muir et al. 2019 and references therein). Furthermore, sea spray aerosols have been shown to be an important mechanism for global transport of PFAS from the marine to coastal terrestrial environments (Johansson et al. 2019).
Arctic communities themselves can be a local source of PFAS in their environment. In Svalbard, firefighting training sites and landfill locations were identified as major PFAS sources (Skaar et al. 2019). Also, at the community of Resolute Bay in the Canadian Arctic, elevated concentrations of PFOS were found in soils near the local airport and former military base, and these were attributed to presence in firefighting foams (Cabrerizo et al. 2018). Similarly, and relative to an area nearby, elevated concentrations of mainly PFOS occurred in the marine environment surrounding a military site in Norway (Landberg et al. 2019). These examples demonstrate the importance of local sources.
The purpose of this study was to illuminate similarities and differences in PFAS levels and patterns in livers from R. tarandus spp. across the Arctic and, where data allowed, also to analyze temporal trends. Information pertaining to PFAS in Russian Rangifer is to our knowledge unavailable and therefore we have focused on North American, Greenland, and European arctic. We analyzed caribou samples from seven areas in northwestern Canada (n = 146) and two areas in southwest Greenland (n = 20). We also analyzed reindeer from Isortoq (south Greenland, n = 10) and Svalbard (n = 7) and three areas in central and northern Sweden (n = 60). We focused on eight PFCA and four PFSA and the precursor PFOSA.
Materials and methods
Sample collection
Altogether, 243 liver samples from caribou and reindeer were analyzed. Information on sampling years, size, and local areas can be found in Table 1 and Fig. 1. PFAS are associated with protein-rich tissues and not lipids. Hence, all concentrations are given based on wet weight (ww) of the samples. Since different laboratories conducted the analyses, the LOD (limit of detection) differ slightly between the studies and we have taken that into account when comparing results.
Caribou from Canada and reindeer from Sweden and Greenland were collected within their respective national programs: Canada’s Northern Contaminants Program, Sweden’s National Monitoring Program (administered by Swedish Museum of Natural History), and the Danish Cooperation for Environment in the Arctic (DANCEA). Samples from Svalbard were collected from local hunters.
Livers from all areas were sampled at harvest and stored at −25 °C until a subsample was collected for analysis. Chemical analyses on the liver samples were conducted at laboratories specific to the country where the samples were collected, i.e., Canada (including southwest Greenland), Denmark (Isortoq, south Greenland samples), Norway, and Sweden. Altogether, 8 PFCA (PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFTeDA, and PFHpA) and 4 PFSA (PFHxS, PFOS, PFDS, and the precursor FOSA) were analyzed within this study. For full names of the acronyms, see Supporting information, Table S1. Portions of the data have been published previously (Bossi et al. 2015; Müller et al. 2011).
Analytical methods
The methods used have been previously published (Müller et al. 2011) and are similar for all samples except those from Isortoq (South Greenland), where an ion-pairing method was used for extraction (Bossi et al. 2015). In general, the liver samples were thawed, and a representative subsample was prepared for acetonitrile-based extractions followed by clean-up with graphite carbon solid-phase cartridges (Canada) or dispersive graphitized carbon (ENVI-Carb) and glacial acetic acid (Sweden and Norway). All laboratories used isotopically labeled (mainly 13C) internal standards (IS) covering a suit of C6–14 PFSA, PFCA, and PFOSA. For the exact IS used in each laboratory, see Lescord et al. (2015); for Canada and southwest Greenland, Carlsson et al. (2014), and Herzke et al. (2009) for Norway; and Eriksson et al. (2016) for Sweden. All laboratories used high-performance liquid chromatography (HPLC) coupled to tandem mass spectrometry (MS/MS) systems with negative electrospray ionization (ESI) for analyses, although there were minor differences in the setup, columns, and brands between the laboratories (see SI Table S2 for a summary of the methods used).
Quantification and quality assurance
The quantification programs and methods, including definitions of, e.g., limits of detection, varied slightly between each sample batch, but are thoroughly described in Carlsson et al. (2014) for Norway, Müller et al. (2011) for Canada and Southwest Greenland, and Bossi et al. (2015) for Isortoq, South Greenland. Samples were quantified with a five (Sweden) or six (Canada) point calibration curve and isotopic dilution method. All laboratories used their internal quality assurance guidelines, as described, and referred below. The Canadian laboratory analyzed certified reference materials (CRM), NIST 1946 Lake Superior Fish Tissue (Reiner et al. 2012), with each batch of 10 samples and the recoveries from certified values of PFOS averaged 113 ± 32% (n = 18). The Norwegian laboratory also analyzed a CRM (PFAS ILS 2011; fish tissue). The CRMs analyzed were within an acceptable range of stated values (±20%). The Swedish laboratory included an in-house quality control sample (fish muscle) in each batch to assess the reproducibility and accuracy (see SI Table 2).
The limit of detection (LOD) was in general defined as a signal-to-noise ratio (S/N) of 3:1 and in the case of detection of target compounds in the blanks, the mean concentration in blank samples with addition of three times the standard deviation was considered LOD. Values of LOD for all compounds and laboratories are provided in Supporting information, Table 1.
All laboratories except for Örebro University in Sweden and NILU in Norway reported total-PFOS(PFOS) whereas Örebro University and NILU reported linear-PFOS (L-PFOS). For the Canadian liver data from 2015, both PFOS and L-PFOS were available (n = 79). For these data, the amount of L-PFOS was ca 64 ± 6% (mean ± SD) of total PFOS. This mean ratio was used to convert the Swedish and Norwegian L-PFOS data to allow comparisons between geographical areas.
Statistical methods
We used regression on order statistics (ROS)(Helsel 2012) to estimate means and medians for each population with less than 80% of the values < LOD. No estimation of means and medians was done if more than 80% of the values were < LOD, following Helsel’s recommendation that the number of censored data should not be above 80% when having sample size <50 (Helsel 2012). Any values below the LOD (non-detections) were replaced by LOD/√2 in cases with maximum of 20% of the samples being less than LOD (Loftis et al. 1989). Prior to statistical analysis, concentration values were log-transformed to approach the assumptions of normal distribution and variance homogeneity. P-values below 0.05 were considered statistically significant and biologically relevant, while P-values between 0.05 and 0.1, albeit insignificant, do not necessarily imply that a biologically relevant tendency was not present.
Analysis of variance (ANOVA) was performed to test for differences in PFAS compounds between the populations. The tests of the explanatory variables were based on Type III Sum of Squares, where each term is evaluated after all factors have been accounted for (i.e., partial, not sequential). Tukey’s post hoc tests for comparisons of means were used to detect significant pairwise differences among predictor variables.
Temporal trends were investigated using those populations that were sampled in multiple years: Porcupine and Qamanirjuaq caribou in Canada (between 2005–2016 and 2008–2016 respectively) and Swedish reindeer (Norrbotten, Västerbotten, and Jämtland, sampled in 2002/2003 and in 2010/2011, see Table 3). Regarding the Porcupine and Qamanirjuaq caribou, temporal trend was analyzed using a robust method, in which annual median concentrations were used as index values. The median was chosen to minimize the influence of outliers and values below detection limits. The method tests for the presence of a log-linear trend and/ornon-linear trend by separating the total variance over time into a log-linear component and a non-linear component (Nicholson et al. 1998). The log-linear trend was tested by log-linear regression. A 3-point running smoother was applied to describe the nonlinear trend component and tested by means of ANOVA. Mood’s median test was applied for each of the Swedish reindeer populations (Norrbotten, Västerbotten and Jämtland) with two sample occasions to test for differences between sampling years. All statistical analyses were performed using R (R Core Team 2019).
Results
Range, mean, and median values for all populations are shown in Table 2. Figure 2 shows boxplots of all populations while Fig. 3 shows the pattern of compounds within each population, Fig. 4 shows time trends for Porcupine caribou, and Fig. S1 in Supporting Information shows time trends for Qamanirjuaq caribou.
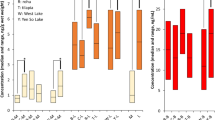
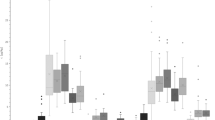
Box plots showing concentrations of PFNA, PFDA, PFUnDA, PFDoDA, PFHxS, and PFOS in fourteen caribou and reindeer populations. The horizontal thick lines show median values. The hinges represent the first and third quantile. The notches represent roughly 95% confidence interval for the median values. Outliers are shown in circles. From left to right: seven populations in Canada (CAN)—Klaza (n = 4), Porcupine (Porc, n = 65), Bluenose West (BN, n = 10), Bathurst (Bat, n = 7), Dolphin & Union (D&U, n = 10), Ahiak (Ahi, n = 10), Qamanirjuaq (Qam, n = 40); three populations in Greenland (GRL)—Akia-Maniitsoq (Akia, n = 10), Kangerlussuaq-Sisimiut (Kanger, n = 10), and Isortoq (SoGr, n = 10). Svalbard, Norway (Sval_NOR, n = 7); and finally three populations in Sweden (SWE)—Norrbotten (Norr, n = 20), Västerbotten (Vastb, n = 20), and Jämtland (Jamt, n = 20)
Time trends for PFDA, PFDoDA, PFTrDA, and PFOS in Porcupine caribou, NW, Canada, show significant yearly decreases of ca 10% except for PFTrDA (only 3% yearly decrease). PFNA and PFUnDA show decreasing trends, however not statistically significant. The solid red line represents a log linear regression and the dashed red line a 3-year smoother. Open circles represent individuals and filled circles are the annual median OBS The titles of the first two graphs are missing! Should read "Porc_CAN PFNA and Porc_CAN PFDA
Age and sex
The caribou and reindeer livers analyzed in this study were collected under varying circumstances. In Sweden, 3-year-old bulls were collected during the annual September slaughter of semi-domestic reindeer. When hunting wild animals (Svalbard, Greenland, Canada), it was not possible to attain the same level of standardized collection regarding season, age, and sex. The mean age of all caribou sampled was 5 years old (range 1–12). Robust analyses of sex with PFAS were not possible. As shown in Table 1, the majority of the collected samples were male. No pronounced relationship with age was observed; hence, those variables were not included in the statistical analyses.
Concentrations of PFAS
Relatively low and similar concentrations of most PFAS were observed in Canada and Isortoq (South Greenland), and for most PFAS also Kangerlussuaq-Sisimiut (Kanger), Svalbard, and Sweden compared to Akia-Maniitsoq(Akia) caribou having elevated concentrations of all PFAS except for PFHxS (see Fig. 2). Elevated concentrations of PFOS were seen in both the Akia and Kanger populations (up to 28 ng/g ww) and in the Swedish populations (up to 14 ng/g ww). All other populations had concentrations below or well below 4 ng/g ww. In contrast, PFHxS showed a different pattern, with Kanger caribou (2.4 ng/g ww) and some individual caribou from Svalbard (up to 0.9 ng/g ww) having the highest concentrations. Other samples were usually below 0.2 ng/g. Concentrations of PFNA were elevated in both populations from Southwest Greenland, in the Akia population up to 35 ng/g (median 21.5 ng/g) and the Kanger population up to 10 ng/g ww (median 8.3 ng/g). In all other populations, the concentrations were most often below 5 ng/g ww (medians between 0.6 and 3.0 ng/g ww).
Furthermore, concentrations of PFDA and PFUnDA were under 5 ng/g in all specimens with the exception of Akia caribou (PFDA up to 17.2 and median 7.8 ng/g and PFUnDA up to 22.4 ng/g and median 11.5 ng/g). As for PFDoDA, all but Akia specimens had concentrations 1.2 ng/g or below, with one exception (one reindeer from Norrbotten, Sweden, had 1.7 ng/g ww). In Akia caribou, the concentrations of PFDoDA were between 1.3 and 4.2 ng/g (median 2.0 ng/g) (Fig. 2).
For central Arctic Canada, a weak spatial trend can be seen with slightly increasing median concentrations of most PFAS from Klaza caribou in the west to Bathurst caribou further east. Also, the Dolphin & Union caribou in the far north generally had very low concentrations, while further east and south, higher concentrations were observed in Ahiak and then Qamanirjuaq caribou (Table 2 and Fig. 2). Within Canada, Bathurst caribou had the highest concentrations of PFOS, PFNA, PFDA, PFUnDA, and PFPoDA.
Relative distribution of PFAS compounds
The pattern of PFAS compounds from caribou/reindeer shifted from PFCA dominated in the Canadian Arctic and western Greenland to PFSA dominated in Svalbard, Sweden, and Isortoq, South Greenland (Fig. 3). The long-chain PFCAs (C9–11; PFNA, PFDA, and PFUnDA) were the most dominant compounds in caribou from Canada, while PFOS dominated samples from Isortoq, Svalbard, and Sweden, followed by PFNA.
Time trends
Temporal trends in several PFAS were evident in caribou from one of the two Canadian populations that was sampled multiple years, as well as the three populations in Sweden (Table 3, Fig. 4 and Fig. S1 in Supporting Information). In Porcupine caribou, concentrations of PFNA, PFDA, PFDoDA, PFUnDA, PFTrDA, and PFOS decreased significantly at a rate of approximately 3–10% annually between 2005 and 2016. PFTrDA first increased and then decreased during the study period, ending at approximately the same level as in the beginning of the time series. An exponential description of the temporal trends was appropriate since non-linear trends components were not significant. As for Qamanirjuaq caribou, no significant trends were seen, likely due to low statistical power with data from only 4 years. Concentrations of PFTrDA increased from mostly below the LOD in 2008 (<0.01) to slightly above the LOD in recent years, including in Qamanirjuaq, indicating an increase in concentrations (median concentration in 2016 was 0.10 ng/g ww, Fig. S1).
Reindeer from the three Swedish populations were sampled on two occasions each, in 2002/2003 and in 2010/2011. Lower concentrations in recent years were observed for PFOS in the two northernmost populations (Norrbotten and Västerbotten, p < 0.001), and a decreasing trend was seen in the southernmost population (Jämtland) (p < 0.07). PFHxS increased in concentrations in the northern population (Norrbotten, p < 0.005) and decreased in Västerbotten (p < 0.003) and Jämtland (p < 0.06). PFNA concentrations increased in Västerbotten reindeer (p < 0.007), although this trend was not observed for the other two populations. No changes in concentrations of PFDA and PFUnDA were observed for any of the three populations. Concentrations of PFDoDA and PFTrDA had too many values below LOD to be able to analyze statistically in the two northern populations (Norrbotten and Västerbotten), and in reindeer from Jämtland, central Sweden, the concentrations were stable.
Discussion
Age and sex
No pronounced relationship was seen between PFAS concentrations, sex, and age in this study. This agrees with several other studies of other mammals (Persson et al. 2013, Roos et al. 2013,Smithwick et al. 2005, Butt et al. 2008, Routti et al. 2016, Shaw et al. 2009, Houde et al. 2006, Kowalczyk et al. 2018).
Concentrations of PFAS
PFOA was found in low concentrations in all study samples. Indeed, all caribou/reindeer had similar or lower concentrations of PFOA than roe deer (Capreolus capreolus) from Germany (Falk et al. 2012) and beavers (Castor fiber) from Poland (et al. 2007). Chamois (Rupicapra rupicapra) from Austria and red fox (Vulpes vulpes) from Germany also had very low concentrations of PFOA, most often below the LOD (Riebe et al. 2016).
Caribou from the two areas in Southwest Greenland, specifically Akia but also Kanger, stand out compared to all other populations in this study by having the highest concentrations of PFOS, PFNA, PFDA, PFUnDA, and PFDoDA. The home range of the Kanger caribou is close to an international airport and a former military base (an American operated base between 1941 and 1992) and the Akia caribou live close to the capital Nuuk. In contrast, the other sampled populations in this study live in relatively remote areas. The proximity to likely local point sources may be an important explanation of the general high concentrations of PFAS (Landberg et al. 2019; Slinde and Høisæter 2017; Stock et al. 2007; Skaar et al. 2019). However, there are marked differences in the pattern of PFAS compounds between the two neighboring Greenland populations. While the PFOA and PFOS concentrations were similar, the Akia caribou had considerably higher concentrations of PFNA, PFDA, PFUnDA, and PFDoDA compared to caribou in Kanger. In contrast, Kanger caribou had higher concentrations of PFHxS. Further, PFHxS showed a different pattern compared to other PFAS. The concentrations were low and similar in Akia caribou, Canada, and Sweden, but levels were somewhat elevated in some Svalbard reindeer and highest in Kanger caribou. The LOD was too high (0.4 ng/g) in samples from reindeer from Isortoq, South Greenland, to allow a comparison. Kanger caribou stand out as having the highest concentrations of PFHxS of any population sampled and much higher than even their neighboring Akia caribou. This may be related to activities at the former American military base, as well as usage of PFHxS during operation of the Kanger International Airport. Besides the proximity to possible local sources, the two areas of Southwest Greenland differ markedly in climate and vegetation types. The Akia caribou live in a wet maritime climate with lots of lichens. The Kanger caribou live in an inland habitat which is a dry continental desert steppe, dominated by dwarf shrub heath and grasslands with almost no lichens (Gamberg et al. 2016). The resulting differences in diets may be a part of the explanation of the differences in contaminant concentrations between these populations. Müller et al. (2011) studied PFAS in a terrestrial food chain in two remote areas of northern Canada and found that the pattern of PFAS differed greatly between vascular plants and lichen. The highest average ΣPFCA concentrations in the two study areas were found in lichen (Cladonia mitis/rangiferina and Flavocetraria nivalis/cucullata) and Arctic willow (Salix pulchra).
Aastrup et al. (2000) observed differences in heavy metal concentrations in caribou from Akia and Kanger and also related that to differences in occurrence of vegetation types and subsequent dietary differences. An additional source for the Akia could be sea spray aerosols which impact grazing areas near open seas (Johansson et al. 2019), which are improbable for inland Kanger. A study analyzing Arctic air samples from Alert (Canada, 2006–2014), Zeppelin (Svalbard, Norway, 2006–2014), and Andøya (Norway, 2010–2014) showed that PFCA were 3 to 30 times higher in the Norwegian samples compared to Alert (Wong et al. 2018). This was explained by the fact that the two Norwegian sites are located closer to the open ocean and more exposed to sea spray aerosol. In the present study, concentrations were generally higher in the European sites compared to Canada, which could be explained by both sea spray aerosol (Akia and Svalbard) and closer proximity to contaminated areas (i.e., human activities) in Sweden and Southwest Greenland.
The median concentrations of PFOS in Akia and Kanger (15 and 14 ng/g) are lower compared to levels in marine mammals in Greenland and Svalbard (Aas et al. 2014; Boisvert et al. 2019; Rigét et al. 2013; Routti et al. 2017; Routti et al. 2019) as well as mammals and aquatic birds from Canada (Martin et al. 2004; Braune et al. 2014). The concentrations of PFOS in this study were highest in Southwest Greenland and Sweden. A somewhat similar circumpolar trend was seen for polar bears, where the lowest concentrations were found in Alaska, higher concentrations further east in Hudson Bay and East Greenland, and the highest in Barents Sea (Routti et al. 2019). However, caribou from Southwest Greenland had much higher concentrations than what was found in livers from terrestrial herbivores in Europe, such as roe deer from Germany sampled the same year (ca 2 ng/g, Falk et al. 2012), and chamois from Austria (2.4 ng/g; Riebe et al. 2016) as well as beaver from Poland (2.4 ng/g; Falandysz et al. 2007), moose (Alces alces) from Norway (range 0.18–0.39, mean 0.27 ng/g ww; Hanssen et al. 2019), and northern Canada (Larter et al. 2017). The median concentration of PFHxS in Kanger caribou (1 ng/g) and Svalbard reindeer (0.3 ng/g) was higher than median concentrations in German roe deer (<0.5 ng/g, Falk et al. 2012), and in beavers from Poland (<0.001 ng/g; Falandysz et al. 2007). The reason for elevated levels of PFHxS in Kanger caribou and some of those from Svalbard is not known, but contamination from local sources, such as firefighting exercises, cannot be ruled out. European chamois and red fox had lower concentrations of PFHxS, most often below the LOD although they are closer to more urban areas (Riebe et al. 2016). The level of PFNA in Akia caribou (mean 21 ng/g) was lower than from polar bears in Greenland but similar to what was found for ringed seals in East Greenland (2011–2012) (Boisvert et al. 2019) and similar to mink and arctic fox (Vulpes lagopus) in Canada (means 16 and 22 ng/g ww, Martin et al. 2004) but higher compared with seals and birds in Canada (means <0.05–5.9 ng/g ww, Martin et al. 2004). In addition, concentrations of PFNA in Akia was much higher compared to European values for roe deer (1.2 ng/g, Falk et al. 2012), chamois, and red fox (1.9 and 1.3 ng/g respectively, Riebe et al. 2016) and beavers (0.12 ng/g, Falandysz et al. 2007). It is not surprising that caribou have lower concentrations compared to animals such as seals and polar bears who are feeding on a higher trophic level, but it is unexpected that they have higher concentration compared to terrestrial mammals specifically herbivores in Europe.
Relative distribution of PFAS compounds
PFNA was the dominant PFAS in caribou from Canada and Southwest Greenland together with PFUnDA and PFDA and found in elevated concentrations in specially Akia. In Canada and Southwest Greenland, PFNA and PFUnDA accounted for usually more than 50% of ΣPFAS, and together with PFDA more than 70%. A similar pattern was seen in moose from the southern northwest territories of Canada, where PFNA was the predominant PFCA (Larter et al. 2017).
In contrast to the present study’s Canadian caribou livers, the most dominant PFCA in five species of birds (eggs) from Arctic Canada were PFUnA and PFTrA, except for black guillemot (Cepphus grylle), where PFDA contributed almost equally with PFTrA to the PFCA profile (Braune and Letcher 2013). In the present study, PFTrA did not contribute significantly to any sample. Previous data has shown an even-odd pattern of PFCA in mammals in remote locations, where by the odd > even for sequential pairs of PFCA as attributed to long range transport of volatile precursors (Martin et al. 2004).
In contrast to caribou from Canada and Southwest Greenland, PFOS was the dominating PFAS in Isortoq (South Greenland), Svalbard, and Sweden. This is more in line with many other studies on PFAS in other wildlife (e.g., Butt et al. 2010; Aas et al. 2014; Falk et al. 2012; Boisvert et al. 2019). However, the situation is changing. Global wildlife sampling over a decade ago uniformly indicated a predominance of PFOS in PFAS congener profiles. However, more recent studies show that long-chain PFCAs are increasing in concentration while PFOS is declining. Therefore, the gap in concentration between long-chainPFCA and PFOS is becoming smaller. For example, Villanger et al. (2020) showed that Svalbard beluga have 14 ng/ml total PFCA and 25 ng/ml PFSA in 2013–14 whereas 15 years earlier the concentrations were 9 ng/ml PFCA and 43 ng/ml PFSA. This difference can be attributed to greater regulatory controls on PFOS in the early 2000s and delayed action on PFCA and their precursors.
Time trends
Concentrations of PFDA, PFDoDA, PFTrDA, and PFOS decreased significantly between 2005 and 2016 in Porcupine caribou (p < 0.05) and a decreasing, not significant, trend was seen for PFNA (p < 0.08) and PFUnDA (p < 0.06). No statistically significant trends were seen in Qamanirjuaq caribou between 2008 and 2016, probably due to low power in the statistical analyses (see Fig. S1 in supporting information). Similarly, concentrations of PFOS in the three Swedish populations were lower in 2010/2011 compared to the early 2000s. Concentrations of PFHxS in reindeer from Norrbotten were higher in 2011 compared to those in 2003 in contrast to the two more southern populations, where the concentrations were lower in 2010 compared to 2002. In Västerbotten, concentrations of PFNA were higher in 2010 compared to those in 2002. For other PFCA, no statistical change in concentrations was observed in the Swedish reindeer. This may be owing to the few years available for temporal trends. Meanwhile, recent studies show that PFAS concentrations in Swedish otters are either still increasing or stable (Roos and Benskin 2016). Other investigations have reported decreasing concentrations of PFOS and other PFAS (Muir et al. 2019; Rigét et al. 2013), as a consequence of the phasing out of PFOS production in USA around 2002 (3M Phase-out plan for POSF-based products, 2000; Giesy and Kannan 2001) as well as the restricted use of PFOS in Europe and Canada shortly thereafter (EC 2006) and the phase out of PFOA by fluorochemical manufacturers in the USA and Europe under the US EPA Stewardship program (USEPA 2006). In East Greenland, annual average concentrations of PFOS in polar bear liver peaked in 2006, at 2966 ng/g ww. The same trend was found for ringed seal liver from both West and East Greenland, where concentrations peaked in 2006 (with a mean of 352 ng/g ww in East Greenland and 397 ng/g in West Greenland, Rigét et al. 2013). A similar pattern was seen in Porcupine caribou in the present study, where concentrations peaked in 2007. Concentrations of PFOS in bird eggs from Prince Leopold Island in Arctic Canada 1975–2012 peaked in 2008 (Braune et al. 2014). Declining trends of PFOS were also observed in ringed seals from the Canadian Arctic over the period 2005 to 2011 (Butt et al. 2010; Muir et al. 2019). The same was seen for many PFCA: increasing concentrations were observed in ringed seals from the Canadian Arctic over the period 1992–2005(Butt et al. 2007) while more recent data show a decline from 2005 to 2010 (Muir et al. 2019), and the same was seen in ringed seals and polar bears from Greenland, where most PFAS peaked in mid 2000s and then decreased (Rigét et al. 2013).
In a time trend study of PFAS in roe deer liver from Germany 1989–2015, concentrations of PFDA, PFOA, and PFNA peaked in 2004–2006, and PFOS already in 1997 (Falk et al. 2019), a decade earlier than what is seen in Porcupine caribou in the present study as well as other biota in the Arctic. This could be expected considering roe deer in Germany probably live closer to contaminated areas; however, it peaked even before the bans of PFOS or even the voluntary cessation of production by 3M in the USA.
Nevertheless, precursors of these PFCA such as side-chain fluorinated polymers are still in many consumer products and may gradually degrade to PFCA. Thus, stable/increasing concentrations are not unexpected (Wang et al. 2015), as observed from Svalbard for arctic foxes, polar bears (Routti et al. 2017), and belugas (Delphinapterus leucas) (Villanger et al. 2020).
Conclusions
The present study compared spatial and temporal PFAS concentrations in caribou/reindeer throughout the western parts of Arctic. In general, similar concentrations of all PFAS compounds were observed in Canada, Svalbard, and Isortoq, south Greenland. Caribou from Southwest Greenland (Akia and Kanger) had the highest concentrations of PFAS. Despite the remoteness, these two Greenland populations had higher PFAS concentrations than roe deer from Germany which feed at a comparable trophic level. Albeit roe deer do not utilize lichens, which caribou are adapted to digest, they are relevant for comparison because the Kanger caribou diet is primarily graminoids owing to absence of suitable lichens in that area. The higher PFAS concentrations observed in Greenland might be due to local sources nearby such as the capital (Akia caribou) as well as the main international airport and an old military base (Kanger caribou). Sea spray aerosols and lichen presence in only Akia could partly explain the differences observed between this population and Kanger caribou.
PFAS showed decreasing temporal trends during the period from early 2000s in Porcupine caribou in Canada, but not in Qamanirjuaq caribou, likely due to few sampling years. However, the same trends were not seen in Sweden, where some PFAS (PFNA, and PFHxS) increased in some populations, but were stable or decreased in the other populations. PFOS decreased in all three areas in Sweden.
The effects of PFAS on terrestrial mammals remain a large data gap in research, as reviewed by Death et al. (2021). While there are a few studies that were designed to investigate bioaccumulation and half-lives of specific PFAS in terrestrial livestock, no overt adverse effects were observed in pigs, cattle, and sheep (Death et al. 2021).
This study has demonstrated that liver concentrations of PFAS in caribou and reindeer are a useful indicator of PFAS entering terrestrial food chains from long-range transport as well as possible local sources. In addition, concentrations and patterns differ among localities, indicating different sources and human activities in different areas. Analysis of more recently collected livers from Akia and Kanger caribou are needed to elucidate the impact of potential local sources. Our study showed the importance of monitoring PFAS in Arctic caribou and reindeer over time since many areas seem to have increasing or stable concentrations despite nearby areas having decreasing concentrations. Currently, the data sets are too small to evaluate these trends in a larger perspective; hence, more analyses from recent years are required. This study has shown that continued monitoring of contaminants in caribou is important, as it provides information on background levels, but also important information for indigenous communities relying on traditional diets.
References
3M Company (2000)Phase-Out Plan for POSF-Based Products. Specialty Materials Markets Group, 3M. St.Paul, MN ; U.S. EPA Docket OPPT-2002-0043
Aas CB, Fuglei E, Herzke D, Yoccoz NG, Routti H (2014) Effect of body condition on tissue distribution of perfluoroalkyl substances (PFASs) in Arctic fox (Vulpes lagopus) [published correction appears in Environ Sci Technol. 2014 Nov 18;48(22):13558]. Environ Sci Technol 48(19):11654–11661
Aastrup P, Rigét F, Dietz R, Asmund G (2000) Lead, zinc, cadmium, mercury, selenium and copper in Greenland caribou and reindeer (Rangifer tarandus). Sci Total Environ 245:149–159
Boisvert G, Sonne C, Rigét F, Dietz R, Letcher RL (2019) Bioaccumulation and biomagnification of perfluoroalkyl acids and precursors in East Greenland polar bears and their ringed seal prey. Environ Pollut 252(Part B):1335–1343
Bossi R, Dam M, Rigét FF (2015) Perfluorinated alkyl substances (PFAS) in terrestrial environments in Greenland and Faroe islands. Chemosphere 129:164–169
Braune BM, Letcher RJ (2013) Perfluorinated sulfonate and carboxylate compounds in eggs of seabirds breeding in the Canadian arctic: temporal trends (1975–2011) and interspecies comparison. Environ Sci Technol 47:616–624
Braune BM, Gaston AJ, Letcher RJ, Grant Gilchrist H, Mallory ML, Provencher JF (2014) A geographical comparison of chlorinated, brominated and fluorinated compounds in seabirds breeding in the eastern Canadian arctic. Environ Res 134:46–56
Budtz-Jørgensen E, Grandjean P (2018) Application of benchmark analysis for mixed contaminant exposures: mutual adjustment of perfluoroalkylate substances associated with immunotoxicity. PLoS One 13(10):e0205388
Butt CM, Muir DCG, Stirling I, Kwan M, Mabury SA (2007) Rapid response of arctic ringed seals to changes in perfluoroalkyl production. Environ Sci Technol 41:42–49
Butt CM, Mabury SA, Kwan M, Wang X, Muir DCG (2008) Spatial trends of perfluoroalkyl compounds in ringed seals (Phoca hispida) from the Canadian arctic. Environ Toxicol Chem 27:542–553
Butt CM, Berger U, Bossi R, Tomy GT (2010) Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci Total Environ 408:2936–2965
Cabrerizo A, Muir DCG, De Silva AO, Wang X, Lamoureux SF, Lafrenière MJ (2018) Legacy and emerging persistent organic pollutants (POPs) in terrestrial compartments in the high arctic: sorption and secondary sources. Environ Sci Technol 52:14187–14197
Carlsson P, Herzke D, Kallenborn R (2014) Polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs) and perfluorinated alkylated substances (PFASs) in traditional seafood items from western Greenland. Environ Sci Pollut Res 21:4741–4750
Cui L, Q-f Z, Liao C-y, J-j F, G-b J (2008) Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch Environ Contam Toxicol 56:338–349
De Witt JC, Peden-Adams MM, Keller JM, Germolec DR (2012) Immunotoxicity of perfluorinated compounds: recent developments. Toxicol Pathol 40:300–311
Death C, Bell C, Champness D, Milne C, Reichman S, Hagen T (2021)Per- and polyfluoroalkyl substances (PFAS) in livestock and game species: a review. Sci Total Environ 774:144795
EC (2006) European Commission Cr. Setting maximum levels for certain contaminants in foodstuffs (text with EEA relevance)
Eriksson U, Roos A, Lind Y, Hope K, Ekblad A, Kärrman A (2016) Comparison of PFASs contamination in the freshwater and terrestrial environments by analysis of eggs from osprey (Pandion haliaetus), tawny owl (Strix aluco), and common kestrel (Falco tinnunculus). Environ Res 149:40–47
Falandysz J, Taniyasu S, Yamashita N, Rostkowski P, Zalewski K, Kannan K (2007) Perfluorinated compounds in some terrestrial and aquatic wildlife species from Poland. J Environ Sci Health A 42:715–719
Falk S, Brunn H, Schröter-Kermani C, Failing K, Georgii S, Tarricone K, Stahl T (2012) Temporal and spatial trends of perfluoroalkyl substances in liver of roe deer (Capreolus capreolus). Environ Pollut 171:1–8
Falk S, Stahl T, Fliedner A, Rüdel H, Tarricone K, Brunn H, Koschorreck J (2019) Levels, accumulation patterns and retrospective trends of perfluoroalkyl acids (PFAAs) in terrestrial ecosystems over the last three decades. Environ Pollut 246:921–931
Gamberg M, Cuyler C, Wang X (2016) Contaminants in two West Greenland caribou populations. Sci Total Environ 554-555:329–336
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35:1339–1342
Hanssen L, Herzke D, Nikiforov V, Moe B, Nygård T, van Dijk J, Gabrielsen GW, Fuglei E, Yeung L, Vogelsang C, Carlsson PM (2019) Screening new PFAS compounds 2018. Norwegian Institute for Air Research, Kjeller, Norway. NILU report no. 23/2019. 96p. ISBN 978–82–425-2992-3
Helsel DR (2012) Statistics for censored environmental data using minitab and r. John Wiley & Sons, Incorporated, New York
Herzke D, Nygård T, Berger U, Huber S, Røv N (2009) Perfluorinated and other persistent halogenated organic compounds in European shag (Phalacrocorax aristotelis) and common eider (Somateria mollissima) from Norway: a suburban to remote pollutant gradient. Sci Total Environ 408:340–348
Houde M, Balmer BC, Brandsma S, Wells RS, Rowles TK, Solomon KR, Muir DCG (2006) Perfluoroalkyl compounds in relation to life-history and reproductive parameters in bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida, USA. Environ Toxicol Chem 25:2405–2412
Houde M, De Silva AO, Muir DCG, Letcher RJ (2011) Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environ Sci Technol 45:7962–7973
Johansson N, Fredriksson A, Eriksson P (2008) Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. NeuroToxicology 29:160–169
Johansson JH, Salter ME, Acosta Navarro JC, Leck C, Nilsson ED, Cousins IT (2019) Global transport of perfluoroalkyl acids via sea spray aerosol. Environ Sci Process Impacts 21:635–649
Kennedy GL, Butenhoff JL, Olsen GW, O'Connor JC, Seakat AM, Perkins RG et al (2004) The toxicology of perfluorooctanoate. Crit Rev Toxicol 34:351–384
Kissa E (2001) Fluorinated surfactants and repellents. Marcel Dekker, New York
Kotthoff M, Müller J, Jürling H, Schlummer M, Fiedler D (2015) Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ Sci Pollut Res 19(22):14546–14559
Kowalczyk J, Numata J, Zimmermann B, Klinger R, Habedank F, Just P, Schafft H, Lahrssen-Wiederholt M (2018) Suitability of wild boar (Sus scrofa) as a bioindicator for environmental pollution with perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS). Arch Environ Contam Toxicol 75:594–606
Landberg A, Breedveld GD, Grønning HM, Kvennås M, Jenssen BM, Hale SH (2019) Bioaccumulation of flurotelomer sulfonates and perfluoroalkyl acids in marine organisms living in aqueous film forming foam (AFFF) impacted waters. Environ Sci Technol 18(53):10951–10960
Larter NC, Muir DCG, Wang X, Allaire D, Kelly A, Cox K (2017) Persistant organic pollutants in the livers of moose harvested in the southern northwest territories, Canada. Alces 53:65–83
Lescord GL, Kidd KA, De Silva AO, Williamson M, Spencer C, Wang X et al (2015) Perfluorinated and polyfluorinated compounds in lake food webs from the Canadian high arctic. Environ Sci Technol 49:2694–2702
Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, Jakobsson K (2018)Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75:46–51
Loftis JC, Ward RC, Phillips RD, Taylor CH (1989) Evaluation of trend detection techniques for use in water quality monitoring programs: U.S. Environmental Protection Agency
Martin JW, Smithwick MM, Braune BM, Hoekstra PF, Muir DCG, Mabury SA (2004) Identification of long-chain perfluorinated acids in biota from the Canadian arctic. Environ Sci Technol 38:373–380
Moody CA, Martin JW, Muir DCG, Mabury SA (2002) Monitoring perfluorinated surfactants in biota and surface water samples following and accidental release of fire-fighting foam into Etobicocke Creek. Environ Sci Technol 36:545–551
Muir D, Bossi R, Carlsson P, Evans M, De Silva A, Halsall C et al (2019) Levels and trends of poly- and perfluoroalkyl substances in the arctic environment - an update. Emerg Contam 5:240–271
Müller CE, De Silva AO, Small J, Williamson M, Wang X, Morris A et al (2011) Biomagnification of perfluorinated compounds in a remote terrestrial food chain: Lichen–caribou–wolf. Environ Sci Technol 45:8665–8673
Nicholson MD, Fryer R, Larsen JR (1998) Temporal trend monitoring: robust method for analyzing contaminant trend monitoring data. (ICES Techniques in Marine Environmental Sciences). Copenhagen, Denmark
OECD (2018) Toward a new comprehensive global database of per- and polyfluoroalkyl substances (PFASs). Health Saftey Publ Ser Risk Manag 39:1–24
Ostertag SK, Tague BA, Humphries MM, Tittlemier SA, Chan HM (2009) Estimated dietary exposure to fluorinated compounds from traditional foods among Inuit in Nunavut, Canada. Chemosphere 75:1165–1172
Persson S, Rotander A, Kärrman A, van Bavel B, Magnusson U (2013) Perfluoroalkyl acids in subarctic wild male mink (Neovison vison) in relation to age, season and geographical area. Environ Int 59:425–430
Petersen MS, Halling J, Jørgensen N, Nielsen F, Grandjean P, Jensen TK, Weihe P (2018) Reproductive function in a population of young Faroese men with elevated exposure to polychlorinated biphenyls (PCBs) and perfluorinated alkylate substances (PFAS). Int J Environ Res Public Health 15:1880
Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH (2006) Sources, fate and transport of perfluorocarboxylates. ChemInform 37
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reiner JL, O’Connell SG, Butt CM, Mabury SA, Small JM, De Silva AO, Muir A, Delinsky D, Strynar MJ, Lindstrom AB, Reagen WK, Malinsky M, Schäfer S, Kwadijk CJAF, Schantz MM, Keller JM (2012) Determination of perfluorinated alkyl acid concentrations in biological standard reference materials. Anal Bioanal Chem 404:2683–2692
Riebe RA, Falk S, Georgii S, Brunn H, Failing K, Stahl T (2016) Perfluoroalkyl acid concentrations in livers of fox (Vulpes vulpes) and chamois (Rupicapra rupicapra) from Germany and Austria. Arch Environ Contam Toxicol 71:7–15
Rigét F, Bossi R, Sonne C, Vorkamp K, Dietz R (2013) Trends of perfluorochemicals in Greenland ringed seals and polar bears: indications of shifts to decreasing trends. Chemosphere 93:1607–1614
Roos A, Benskin J (2016) Perfluorerade ämnen i utter från Sverige 1970-2015. Report from SMNH 1:2016 in Swedish
Roos A, Berger U, Järnberg U, van Dijk J, Bignert A (2013) Increasing concentrations of perfluoroalkyl acids in Scandinavian otters (Lutra lutra) between 1972 and 2011: a new threat to the otter population? Environ Sci Technol 47:11757–11765
Routti H, Gabrielsen GW, Herzke D, Kovacs KM, Lydersen C (2016) Spatial and temporal trends in perfluoroalkyl substances (PFASs) in ringed seals (Pusa hispida) from Svalbard. Environ Pollut 214:230–238
Routti H, Aars J, Fuglei E, Hanssen L, Lone K, Polder A, Pedersen ÅØ, Tartu S, Welker JM, Yoccoz NG (2017) Emission changes dwarf the influence of feeding habits on temporal trends of per- and polyfluoroalkyl substances in two Arctic top predators. Environ Sci Technol 20(51):11996–12006
Routti H, Atwood TC, Bechshoft T, Boltunov A, Ciesielski TM, Desforges JP, Dietz R, Gabrielsen GW, Jenssen BM, Letcher RJ, McKinney MA, Morris AD, Rigét FR, Sonne S, Styrishave B, Tartu S (2019) State of knowledge on current exposure, fate and potential health effects of contaminants in polar bears from the circumpolar Arctic. Sci Total Environ 664:1063–1083
Schiavone A, Corsolini S, Kannan K, Tao L, Trivelpiece W, Torres D et al (2009) Perfluorinated contaminants in fur seal pups and penguin eggs from South Shetland, Antarctica. Sci Total Environ 407:3899–3904
Seacat AM, Thomford PJ, Hansen KJ, Clemen LA, Eldridge SR, Elcombe CR, Butenhoff JL (2003)Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology 183:117–131
Shaw S, Berger ML, Brenner D, Tao L, Wu Q, Kannan K (2009) Specific accumulation of perfluorochemicals in harbor seals (Phoca vitulina concolor) from the Northwest Atlantic. Chemosphere 74:1037–1043
Skaar J S, Ræder EM, Lyche JL, Ahrens L, Kallenborn R (2019) Elucidation of contamination sources for poly- and perfluoroalkyl substances (PFASs) on Svalbard (Norwegian Arctic). 2019. Environ Sci Pollut Res 26:7356–7363. https://doi.org/10.1007/s11356-018-2162-4
Slinde GA, Høisæter Å (2017) Source tracing of PFAS to Tyrifjorden - final report. Norwegian Environment Agency
Smithwick M, Mabury SA, Solomon KR, Sonne C, Martin JW, Born EW, Dietz R, Derocher AE, Letcher RJ, Evans TJ, Gabrielsen GW, Nagy J, Stirling I, Taylor MK, Muir DCG (2005) Circumpolar study of perfluoroalkyl contaminants in polar bears (Ursus maritimus). Environ Sci Technol 39:5517–5523
Stock NL, Furdui VI, Muir DCG, Mabury SA (2007) Perfluoroalkyl contaminants in the Canadian Arctic: evidence of atmospheric transport and local contamination. Environ Sci Technol 41:3529–3536
US EPA (2006) PFOA Stewardship Program docket ID number EPA-HQ-OPPT-2006-0621. US Environmental Protection Agency. Washington, DC
Villanger GD, Kovacs KM, Lydersen C, Haug LS, Sabaredzovic A, Jenssen BM, Routti H (2020) Perfluoroalkyl substances (PFASs) in white whales (Delphinapterus leucas) from Svalbard a comparison of concentrations in plasma sampled 15 years apart. Environ Pollut 263:114497. https://doi.org/10.1016/j.envpol.2020.114497
Wang Z, Cousins IT, Scheringer M, Hungerbuehler K (2013) Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ Int 60:242–248
Wang Z, Cousins IT, Scheringer M, Hungerbuehler K (2015) Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFASs) and their precursors: status quo, ongoing challenges and possible solutions. Environ Int 75:172–179
Wong F, Shoeib M, Katsoyiannis A, Eckhardt S, Stohl A, Bohlin-Nizzetto P, Li H, Fellin P, Su Y, Hung H (2018) Assessing temporal trends and source regions of per- and polyfluoroalkyl substances (PFASs) in air under the Arctic Monitoring and Assessment Programme (AMAP). Atmos Environ 172:65–73
Xie S, Wang T, Liu S, Jones KC, Sweetman AJ, Lu Y (2013) Industrial source identification and emission estimation of perfluorooctane sulfonate in China. Environ Int 52:1–8
Acknowledgements
We want to thank all the hunters who supplied samples. Mary Williamson (ECCC, Burlington ON) is acknowledged for sample analysis of the Canadian and Southwest Greenland samples and Angela Milani for aging Canadian caribou teeth. The analyses of caribou samples from Southern Greenland was conducted by the Danish Cooperation for Environment in the Arctic (DANCEA). The analyses of caribou samples from Southwest Greenland were funded by CARMA (CircumArctic Rangifer Monitoring and Assessment Network). The collection and analysis of the Swedish reindeers was funded by the Swedish Environmental Protection Agency. Several people have been involved in sampling, preparing samples, and performing analyses in laboratories and in the field. Finally, we want to thank Dr. Amila De Silva for excellent comments on the manuscript.
Availability of data and materials
The datasets used during the current study can be made available from the corresponding author or chemist on reasonable request.
Funding
Open access funding provided by Swedish Museum of Natural History. The analyses of caribou samples Southwest Greenland were funded by CARMA (CircumArctic Rangifer Monitoring and Assessment Network). The collection and analysis of Swedish reindeer samples was financed by the Swedish Environmental Protection Agency. Collection of Svalbard samples were done by local hunters and the analyses were financed by Svalbard Environmental Fund (project no. 10/48). The Canadian populations were monitored under the Northern Contaminants Program (Crown-Indigenous Relations and Northern Affairs Canada), Environment and Climate Change Canada (ECCC) and the Government of Northwest Territories (Department of Resources, Wildlife, and Economic Development).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analyses were performed by all authors of the manuscript. In detail: collection of samples in the field—M. G., C. C., F. R., P. C., A. R., and Y. L. Performed the chemical analyses: D. M., A. K., R. B., P. C. Performed statistical analyses: F. R. The first draft of the manuscript was written by Anna Roos and all authors commented on previous versions of the manuscript. All authors read and approved.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Roland Peter Kallenborn
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
•Elevated concentrations of PFAS in Southwest Greenland.
•PFOS predominates in Scandinavia and parts of Greenland.
•PFNA and PFUnDA predominates in Canada.
•Some PFAS showed decreasing time trends.
•PFHxS increased in concentrations in remote Northern Sweden reindeer.
•PFNA increased in concentrations in Central Sweden reindeer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roos, A.M., Gamberg, M., Muir, D. et al. Perfluoroalkyl substances in circum-ArcticRangifer: caribou and reindeer. Environ Sci Pollut Res 29, 23721–23735 (2022). https://doi.org/10.1007/s11356-021-16729-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16729-7