Abstract
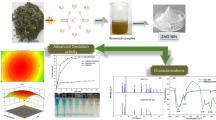
Water contamination due to release of dye containing effluents is one of the environmental problems of serious concern today. The present study investigate the green synthesis of zinc oxide nanoparticles (ZnO-NPs) doped on activated carbon (AC) prepared from walnut peel extract and to estimate its efficiency in the removal of Eosin Y (Eo-Y) and Erythrosine B (Er-B) from its aqueous solution. The synthesized AC-ZnO was identified by field emission scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), and the Brunauer–Emmett–Teller. The influence of various parameters such as pH, dosage of AC-ZnO, contact time, and concentrations of Eo-Y and Er-B was also studied. The pH 3 was observed as the optimum pH while the equilibrium was noticed to reach in 30 min at dosage of 1 g/L and initial concentration 100 mg/L for Eo-Y and Er-B adsorption onto AC-ZnO. The maximum adsorption capacity of Eo-Y and Er-B onto AC-ZnO was found to be 163.9 and 144.92 mg/g (and removal efficiencies of 95.11 and 98.31 %), respectively. The process of Eo-Y and Er-B adsorption on AC-ZnO was observed to be depended on the pseudo-second-order kinetic model which indicates chemisorption processes. Langmuir adsorption isotherm model test described the removal of Eo-Y and Er-B on AC-ZnO. The thermodynamic data indicated that the adsorption was endothermic process. Also, the values, SBET and VTOTAL, for the AC-ZnO were equal to 725.65 m2/g and 0.6004 cm3/g, respectively. The results of this study exhibited that AC-ZnO was a very effective method that can be used for the removal of Eo-Y and Er-B from aqueous solutions.





Similar content being viewed by others
Data Availability
The data used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abdollahzadeh H, Fazlzadeh M, Afshin S, Arfaeinia H et al. (2020) Efficiency of activated carbon prepared from scrap tires magnetized by Fe3O4 nanoparticles: characterisation and its application for removal of reactive blue19 from aquatic solutions. International Journal of Environmental Analytical Chemistry, 1-15
Acisli O, Acar I, Khataee A (2020) Preparation of a fly ash-based geopolymer for removal of a cationic dye: isothermal, kinetic and thermodynamic studies. J Ind Eng Chem 83:53–63
Adelkhani H, Ghaemi M, Ruzbehani M (2011) Evaluation of the porosity and the nano-structure morphology of MnO2 prepared by pulse current electrodeposition. Int J Electrochem Sci 6:123–135
Afshina S, Rashtbaria Y, Shirmardic M, Vosoughib M et al (2019) Adsorption of Basic Violet 16 dye from aqueous solution onto mucilaginous seeds of Salvia sclarea: kinetics and isotherms studies. Desalin Water Treat 161:365–375
Ahmadi S, Igwegbe CA, Rahdar S, Asadi Z (2019) The survey of application of the linear and nonlinear kinetic models for the adsorption of nickel(II) by modified multi-walled carbon nanotubes. Applied Water Science 9
Ahmadi S, Mohammadi L, Rahdar A, Rahdar S, Dehghani R, Adaobi Igwegbe C, Kyzas GZ (2020) Acid dye removal from aqueous solution by using neodymium(III) oxide nanoadsorbents. Nanomaterials (Basel) 10
Al-Degs YS, Abu-El-Halawa R, Abu-Alrub SS (2012) Analyzing adsorption data of erythrosine dye using principal component analysis. Chem Eng J 191:185–194
Alencar WS, Lima EC, Royer B, dos Santos BD, Calvete T, da Silva EA, Alves CN (2012) Application of aqai stalks as biosorbents for the removal of the dye Procion Blue MX-R from aqueous solution. Sep Sci Technol 47:513–526
Ali I, Afshinb S, Poureshgh Y et al (2020) Green preparation of activated carbon from pomegranate peel coated with zero-valent iron nanoparticles (nZVI) and isotherm and kinetic studies of amoxicillin removal in water. Environ Sci Pollut Res 27:36732–36743. https://doi.org/10.1007/s11356-020-09310-1
Al-Qodah Z, Yahya MA, Al-Shannag M (2017) On the performance of bioadsorption processes for heavy metal ions removal by low-cost agricultural and natural by-products bioadsorbent: a review. Desalin Water Treat 85:339–357
Al-Shawabkah R, Al-Qodah Z, Al-Bsoul A (2015) Bio-adsorption of triadimenol pesticide from aqueous solutions using activated sludge of dairy plants. Desalin Water Treat 53:2555–2564
Ansari F, Ghaedi M, Taghdiri M, Asfaram A (2016) Application of ZnO nanorods loaded on activated carbon for ultrasonic assisted dyes removal: experimental design and derivative spectrophotometry method. Ultrason Sonochem 33:197–209
Arabi SMS, Lalehloo RS, Olyai MRTB, Ali GA et al (2019) Removal of congo red azo dye from aqueous solution by ZnO nanoparticles loaded on multiwall carbon nanotubes. Physica E: Low-dimensional Systems and Nanostructures 106:150–155
Baghapour MA, Jahed B, Joshani GH (2013) Preparation of activated carbon from waste tires and its application in gasoline removal from water. Iranian Journal of Health and Environment 6
Balarak D, Mahdavi Y, Bazrafshan E, Mahvi AH et al (2016) Adsorption of fluoride from aqueous solutions by carbon nanotubes: determination of equilibrium, kinetic, and thermodynamic parameters. Fluoride 49:71
Carmen Apostol L, Ghinea C, Alves M, Gavrilescu M (2016) Removal of Erythrosine B dye from water effluents using crop waste pumpkin seed hulls as adsorbent. Desalin Water Treat 57:22585–22608
Chatterjee S, Chatterjee S, Chatterjee BP, Das AR, Guha AK (2005) Adsorption of a model anionic dye, eosin Y, from aqueous solution by chitosan hydrobeads. J Colloid Interface Sci 288:30–35
Chieng HI, Lim LB, Priyantha N (2015) Sorption characteristics of peat from Brunei Darussalam for the removal of rhodamine B dye from aqueous solution: adsorption isotherms, thermodynamics, kinetics and regeneration studies. Desalin Water Treat 55:664–677
Chigozie UF, Joseph NT (2014) Removal of Orange-G, Vat Yellow, Erythrosine dyes from synthetic wastewater by electrocoagulation and nanofiltration. J Adv Chem Eng 4:2
Côrtes LN, Druzian SP, Streit AFM et al (2019) Preparation of carbonaceous materials from pyrolysis of chicken bones and its application for fuchsine adsorption. Environ Sci Pollut Res 26:28574–28583
Eletta OA, Adeniyi AG, Ighalo JO, Onifade DV et al (2020) Valorisation of cocoa (Theobroma cacao) pod husk as precursors for the production of adsorbents for water treatment. Environmental Technology Reviews 9:20–36
Elhami S, Abrishamkar M, Esmaeilzadeh L (2013) Preparation and characterization of diethylentriamine-montmorillonite and its application for the removal of Eosin Y dye: optimization, kinetic and isotherm studies
Fazlzadeh M, Khosravi R, Zarei A (2017) Green synthesis of zinc oxide nanoparticles using Peganum harmala seed extract, and loaded on Peganum harmala seed powdered activated carbon as new adsorbent for removal of Cr (VI) from aqueous solution. Ecol Eng 103:180–190
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57:1100–1107
Ghaedi M, Ghayedi M, Kokhdan SN, Sahraei R, Daneshfar A (2013) Palladium, silver, and zinc oxide nanoparticles loaded on activated carbon as adsorbent for removal of bromophenol red from aqueous solution. J Ind Eng Chem 19:1209–1217
Ghosh D, Bhattacharyya KG (2002) Adsorption of methylene blue on kaolinite. Appl Clay Sci 20:295–300
Gupta VK, Mittal A, Kurup L, Mittal J (2006) Adsorption of a hazardous dye, erythrosine, over hen feathers. J Colloid Interface Sci 304:52–57
Gupta V, Gupta B, Rastogi A, Agarwal S et al (2011) A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye—Acid Blue 113. J Hazard Mater 186:891–901
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Igwegbe C, Al-Rawajfeh A, Al-Itawi HI, Sharadqah S et al (2019a) Utilization of calcined gypsum in water and wastewater treatment: removal of phenol. Journal of Ecological Engineering 20:1–10
Igwegbe CA, Mohmmadi L, Ahmadi S, Rahdar A, Khadkhodaiy D, Dehghani R, Rahdar S (2019b) Modeling of adsorption of Methylene Blue dye on Ho-CaWO4 nanoparticles using response surface methodology (RSM) and artificial neural network (ANN) techniques. MethodsX 6:1779–1797
Jamshidi M, Ghaedi M, Dashtian K, Ghaedi A et al (2016) Highly efficient simultaneous ultrasonic assisted adsorption of brilliant green and eosin B onto ZnS nanoparticles loaded activated carbon: artificial neural network modeling and central composite design optimization. Spectrochim Acta A Mol Biomol Spectrosc 153:257–267
Khosravi R, Fazlzadehdavil M, Barikbin B, Hossini H (2015) Electro-decolorization of Reactive Red 198 from aqueous solutions using aluminum electrodes systems: modeling and optimization of operating parameters. Desalin Water Treat 54:3152–3160. https://doi.org/10.1080/19443994.2014.913204
Khosravi R, Hazrati S, Fazlzadeh M (2016) Decolorization of AR18 dye solution by electrocoagulation: sludge production and electrode loss in different current densities. Desalin Water Treat 57:14656–14664. https://doi.org/10.1080/19443994.2015.1063092
Kıvanç MR, Yönten V (2020) A statistical optimization of methylene blue removal from aqueous solutions by Agaricus Campestris using multi-step experimental design with response surface methodology: isotherm, kinetic and thermodynamic studies. Surfaces and interfaces 18:100414
Lagergren S, Svenska B (1898) On the theory of so-called adsorption of materials. Royal Swed Acad Sci Doc 24:1–13
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Leili M, Fazlzadeh M, Bhatnagar A (2018) Green synthesis of nano-zero-valent iron from Nettle and Thyme leaf extracts and their application for the removal of cephalexin antibiotic from aqueous solutions. Environmental Technology (United Kingdom) 39:1158–1172. https://doi.org/10.1080/09593330.2017.1323956
Liu Y, Liu Y-J (2008) Biosorption isotherms, kinetics and thermodynamics. Sep Purif Technol 61:229–242
Liu Y, Huang Y, Xiao A, Qiu H, Liu L (2019) Preparation of magnetic Fe3O4/MIL-88A nanocomposite and its adsorption properties for bromophenol blue dye in aqueous solution. Nanomaterials 9:51
Mahvi AH, Rahdar A, Igwegbe CA, Rahdar S et al. (2020) Fluoride removal from aqueous solutions by zinc oxide nanoparticles. Fluoride
Manickam JR (2016) Study of water soluble dyes adsorption from aqueous solution by Prosopis spicigera L. wood (PSLW) carbon. Indian Journal of Chemical Technology (IJCT) 23:22–30
Mansour F, Al-Hindi M, Yahfoufi R, Ayoub GM et al (2018) The use of activated carbon for the removal of pharmaceuticals from aqueous solutions: a review. Rev Environ Sci Biotechnol 17:109–145
Mendez-Paz D, Omil F, Lema J (2005) Anaerobic treatment of azo dye Acid Orange 7 under fed-batch and continuous conditions. Water Res 39:771–778
Mohebbi P, Parvini M, Mousav H (2014) Removal of erythrosine dyes from aquatic environment using Ziziphus nummularia kernel. Iran J Energy Environ 5:400–406
Mouni L, Belkhiri L, Bollinger J-C, Bouzaza A, Assadi A, Tirri A, Dahmoune F, Madani K, Remini H (2018) Removal of Methylene Blue from aqueous solutions by adsorption on kaolin: kinetic and equilibrium studies. Appl Clay Sci 153:38–45
Obiora-Okafo I, Onukwuli O, Eli-Chukwu N (2020) Evaluation of bio-coagulants for colour removal from dye synthetic wastewater: characterization, adsorption kinetics, and modelling approach. Water SA 46:300–312
Ong S, Lee C, Zainal Z (2007) Removal of basic and reactive dyes using ethylenediamine modified rice hull. Bioresour Technol 98:2792–2799
Oyelude EO, Awudza JA, Twumasi SK (2017) Equilibrium, kinetic and thermodynamic study of removal of eosin yellow from aqueous solution using teak leaf litter powder. Sci Rep 7:12198
Porkodi K, Kumar KV (2007) Equilibrium, kinetics and mechanism modeling and simulation of basic and acid dyes sorption onto jute fiber carbon: eosin yellow, malachite green and crystal violet single component systems. J Hazard Mater 143:311–327
Ramezani F, Kazemi B, Jebali A (2013) Biosynthesis of silver nanoparticles by Leishmania sp. New Cellular and Molecular Biotechnology Journal 3:107–111
Rashtbari Y, Afshin S, Hamzezadeh A, Abazari M et al (2019) Application of powdered activated carbon coated with zinc oxide nanoparticles prepared using a green synthesis in removal of Reactive Blue 19 and Reactive Black-5: adsorption isotherm and kinetic models. Desalin Water Treat 179:354–367
Rashtbari Y, Hazrati S, Azari A, Afshin S, Fazlzadeh M, Vosoughi M (2020) A novel, eco-friendly and green synthesis of PPAC-ZnO and PPAC-nZVI nanocomposite using pomegranate peel: cephalexin adsorption experiments, mechanisms, isotherms and kinetics. Adv Powder Technol 31:1612–1623
Regti A, Laamari MR, Stiriba S-E, El Haddad M (2017) Use of response factorial design for process optimization of basic dye adsorption onto activated carbon derived from Persea species. Microchem J 130:129–136
Rivera-Utrilla J, Sánchez-Polo M, Prados-Joya G, Ferro-García M et al (2010) Removal of tinidazole from waters by using ozone and activated carbon in dynamic regime. J Hazard Mater 174:880–886
Saif MMS, Kumar NS, Prasad M (2012) Binding of cadmium to Strychnos potatorum seed proteins in aqueous solution: adsorption kinetics and relevance to water purification. Colloids Surf B: Biointerfaces 94:73–79
Seid-Mohammadi A, Shabanloo A, Fazlzadeh M, Poureshgh Y (2017) Degradation of acid blue 113 by US/H2O2/Fe2+ and US/S2O8 2– /Fe2+ processes from aqueous solutions. Desalin Water Treat 78:273–280
Shi X, Zhang S, Chen X, Mijowska E (2019) Evaluation of nanoporous carbon synthesized from direct carbonization of a metal–organic complex as a highly effective dye adsorbent and supercapacitor. Nanomaterials 9:601
Shokoohi R, Samadi MT, Amani M, Poureshgh Y (2018) Modeling and optimization of removal of cefalexin from aquatic solutions by enzymatic oxidation using experimental design. Braz J Chem Eng 35:943–956
Silva TL, Cazetta AL, Souza PSC, Zhang T, Asefa T, Almeida VC (2018) Mesoporous activated carbon fibers synthesized from denim fabric waste: efficient adsorbents for removal of textile dye from aqueous solutions. J Clean Prod 171:482–490
Sonwani RK, Swain G, Giri BS, Singh RS, Rai BN (2020) Biodegradation of Congo red dye in a moving bed biofilm reactor: Performance evaluation and kinetic modeling. Bioresource Technology 302:122811
Streit AF, Côrtes LN, Druzian SP, Godinho M et al (2019) Development of high quality activated carbon from biological sludge and its application for dyes removal from aqueous solutions. Sci Total Environ 660:277–287
Sun JD, Henderson RF, Marshall TC, Cheng Y-S, Dutcher JS, Pickrell JA, Mauderly JL, Hahn FF, Banas DA, Seiler FA (1987) The inhalation toxicity of two commercial dyes: solvent yellow 33 and solvent green 3. Fundam Appl Toxicol 8:358–371
Tan TCN, Sen TK (2020) Aqueous-phase methylene blue (MB) dye removal by mixture of eucalyptus bark (EB) biomass and kaolin clay (KC) adsorbents: kinetics, thermodynamics, and isotherm modeling. Sep Sci Technol 55:1036–1050
Tarkwa J-B, Oturan N, Acayanka E, Laminsi S, Oturan MA (2019) Photo-Fenton oxidation of Orange G azo dye: process optimization and mineralization mechanism. Environ Chem Lett 17:473–479
Venkatesh S, Venkatesh K (2020) Ozonation for degradation of acid red 14: effect of buffer solution. Proceedings of the National Academy of Sciences, India Section A: Physical Sciences 90:209–212
Xiao J, Gao B, Yue Q, Gao Y, Li Q (2015) Removal of trihalomethanes from reclaimed-water by original and modified nanoscale zero-valent iron: characterization, kinetics and mechanism. Chem Eng J 262:1226–1236
Zalloum HM, Al-Qodah Z, Mubarak MS (2008) Copper adsorption on chitosan-derived Schiff bases. J Macromol Sci A 46:46–57
Zhang H, Duan L, Zhang Y, Wu F (2005) The use of ultrasound to enhance the decolorization of the CI Acid Orange 7 by zero-valent iron. Dyes Pigments 65:39–43
Funding
This research work was financially supported by Ardabil University of Medical Sciences; we gratefully acknowledge them.
Author information
Authors and Affiliations
Contributions
Yousef Poureshgh, Yousef Rashtbari, and Mehdi Fazlzadeh participated in the conceptualization and design of the research and supervised the work. Shirin Afshin and Asghar Hamzezadeh are responsible for experimental analysis and interpretation of data. Abdolmajid Gholizadeh contributed to literature search and quality assessment. All authors have read and approved the final paper as submitted
Corresponding authors
Ethics declarations
Ethical approval
The protocol was approved by the Institutional Review Board of Ardabil University of Medical Sciences (Approval ID: IR.ARUMS.REC.1398.015).
Consent to participate
Not applicable.
Consent for publication
All the authors agreed to publish the data in this journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rashtbari, ., Afshin, S., Hamzezadeh, A. et al. Green synthesis of zinc oxide nanoparticles loaded on activated carbon prepared from walnut peel extract for the removal of Eosin Y and Erythrosine B dyes from aqueous solution: experimental approaches, kinetics models, and thermodynamic studies. Environ Sci Pollut Res 29, 5194–5206 (2022). https://doi.org/10.1007/s11356-021-16006-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16006-7