Abstract
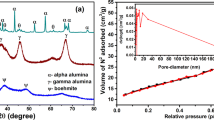
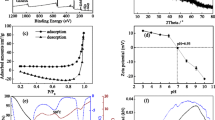
This work introduced a new way of fabricating a granular material with the supply of Al-rich precipitates selectively obtained from acid mine drainage (AMD), and its potential as a promising adsorbent for fluoride (F) was evaluated. Through the selective sequential precipitation (SP) process in the field, Al-rich precipitates with high purity (>81%) were collected at the high recovery rate (>99.8%) as a raw material for adsorbent fabrication. The granular adsorbent (ALB) was synthesized through encapsulation of precipitate powders by chemically inducing polymeric bead formation. The characterization results revealed that ALB possessed a highly porous structure and embedded a large number of nanoparticles of amorphous Al hydroxides inside its framework. Less adsorption of F occurred at an alkaline pH condition due to the competitive effect of hydroxyl ions. The adsorption process can be divided into fast adsorption by the outer surface and slow diffusion in the inner phase. The maximum adsorption capacity of ALB for F was calculated to be 17.7 mg g−1 in the Langmuir isotherm model fitting results. By the repetitive adsorption/desorption and XPS results, it turned out that both chemisorption and physisorption gave a contribution in the removal of F, and the regeneration of adsorbent using NaOH was effective to restore the adsorption capability but accompanied the loss of adsorption sites. As a result, it can be concluded that a granule-type material fabricated using Al-rich precipitates selectively recovered from AMD neutralization can be considered as a promising adsorbent for F removal in aqueous solution.









Similar content being viewed by others
References
Alsaiari A, Tang HL (2018) Field investigations of passive and active processes for acid mine drainage Treatment: are anions a concern? Ecol Eng 122:100–106
Amaya-Vias D, Tataru L, Herce-Sesa B, Lopez-Lopez JA, Lopez-Ramirez JA (2019) Metals removal from acid mine drainage (Tinto River, SW Spain) by water gap and air gap membrane distillation. J Membrane Sci 582:20–29
Ayinde WB, Gitari WM, Munkombwe M, Samie A, Smith JA (2020) Green synthesis of AgMgOnHaP nanoparticles supported on chitosan matrix: defluoridation and antibacterial effects in groundwater. J Environ Chem Eng 8(5):104026. https://doi.org/10.1016/j.jece.2020.104026
Bok S, Yim G, Oh C, Ji S, Cheong Y, Baek K (2017) Treatment of selective sequential precipitation for recovering Fe and Al from mine water of abandoned coal mine. J Korean Soc Miner Energy Resour Eng 54:215–222
Cho DW, Jeon BH, Jeong Y, Nam IH, Choi UK, Kumar R, Song H (2016) Synthesis of hydrous zirconium oxide-impregnated chitosan beads and their application for removal of fluoride and lead. Appl Surf Sci 372:13–19
Cho D-W, Han Y-S, Lee J, Jang J-Y, Yim G, Cho S, Lee J-S, Cheong Y-W (2020) Water defluorination using granular composite synthesized via hydrothermal treatment of polyaluminum chloride (PAC) sludge. Chemosphere 247:125899
Du XL, Wang YQ, Su XH, Li JG (2009) Influences of pH value on the microstructure and phase transformation of aluminum hydroxide. Powder Technol 192:40–46
Eggermont SGF, Rua-Ibarz A, Tirez K, Dominguez-Benetton X, Fransaer J (2019) Oxidation-assisted alkaline precipitation: the effect of H2O2 on the size of CuO and FeOOH nanoparticles. Rsc Adv 9:29902–29908
Fan C, Guo CL, Zeng YF, Tu ZH, Ji YP, Reinfelder JR, Chen MQ, Huang WL, Lu GN, Yi XY, Dang Z (2019) The behavior of chromium and arsenic associated with redox transformation of schwertmannite in AMD environment. Chemosphere 222:945–953
Feng GR, Ma JC, Zhang XP, Zhang QF, Xiao YQ, Ma QL, Wang SB (2019) Magnetic natural composite Fe3O4-chitosan@bentonite for removal of heavy metals from acid mine drainage. J Colloid Interf Sci 538:132–141
Flores RG, Andersen SLF, Maia LKK, Jose HJ, Moreira RDPM (2012) Recovery of iron oxides from acid mine drainage and their application as adsorbent or catalyst. J Environ Manage 111:53–60
Hristovski KD, Markovski J (2017) Engineering metal (hydr)oxide sorbents for removal of arsenate and similar weak-acid oxyanion contaminants: a critical review with emphasis on factors governing sorption processes. Sci Total Environ 598:258–271
Jouini M, Neculita CM, Genty T, Benzaazoua M (2020) Environmental behavior of metal-rich residues from the passive treatment of acid mine drainage. Sci Total Environ 712:136541
Kim Y (2018) Effects of different oxyanions in solution on the precipitation of jarosite at room temperature. J Hazard Mater 353:118–126
Kumar E, Bhatnagar A, Ji M, Jung W, Lee SH, Kim SJ, Lee G, Song H, Choi JY, Yang JS, Jeon BH (2009) Defluoridation from aqueous solutions by granular ferric hydroxide (GFH). Water Res 43:490–498
Li TG, Xie DL, He CH, Xu XJ, Huang B, Nie R, Liu SL, Duan ZY, Liu W (2016a) Simultaneous adsorption of fluoride and hexavalent chromium by synthetic mesoporous alumina: performance and interaction mechanism. Rsc Adv 6:48610–48619
Li XX, Wu P, Han ZW, Shi JF (2016b) Sources, distributions of fluoride in waters and its influencing factors from an endemic fluorosis region in central Guizhou, China. Environ Earth Sci 75:981
Liu F, Zhang WF, Tao L, Hao BY, Zhang J (2019) Simultaneous photocatalytic redox removal of chromium(VI) and arsenic(III) by hydrothermal carbon-sphere@nano-Fe3O4. Environ Sci-Nano 6:937–947
Macingova E, Luptakova A (2012) Recovery of metals from acid mine drainage. Chem Eng Trans 28:109–114
Meighan M, MacNeil J, Falconer R (2008) Determining the solubility product of Fe(OH)(3): an equilibrium study with environmental significance. J Chem Educ 85:254–255
Moodley I, Sheridan CM, Kappelmeyer U, Akcil A (2018) Environmentally sustainable acid mine drainage remediation: research developments with a focus on waste/by-products. Miner Eng 126:207–220
Morgan B, Lahav O (2007) The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O-2 in aqueous solution—basic principles and a simple heuristic description. Chemosphere 68:2080–2084
Murambasvina GMC (2020) Effective fluoride adsorption using water hyacinth beads doped with hydrous oxides of aluminium and iron. Groundwater Sus Devel 10:100302
Naidu G, Ryu S, Thiruvenkatachari R, Choi Y, Jeong S, Vigneswaran S (2019) A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ Pollut 247:1110–1124
Nekhunguni PM, Tavengwa NT, Tutu H (2017) Investigation of As(V) removal from acid mine drainage by iron (hydr) oxide modified zeolite. J Environ Manage 197:550–558
Nigri EM, Cechinel MAP, Mayer DA, Mazur LP, Loureiro JM, Rocha SDF, Vilar VJP (2017) Cow bones char as a green sorbent for fluorides removal from aqueous solutions: batch and fixed-bed studies. Environ Sci Pollut R 24:2364–2380
Oh C, Bok S, Yim G, Cheong Y, Ji S (2018) An investigation into precipitate behaviour for effective operation of settling tanks through selective precipitation. Water Environ J 32:527–536
Peak D (2006) Adsorption mechanisms of selenium oxyanions at the aluminum oxide/water interface. J Colloid Interf Sci 303:337–345
Periyasamy S, Gopalakannan V, Viswanathan N (2018) Hydrothermal assisted magnetic nano-hydroxyapatite encapsulated alginate beads for efficient Cr(VI) uptake from water. J Environ Chem Eng 6:1443–1454
Seo EY, Cheong YW, Yim GJ, Min KW, Geroni JN (2017) Recovery of Fe, Al and Mn in acid coal mine drainage by sequential selective precipitation with control of pH. Catena 148:11–16
Tan LC, Papirio S, Luongo V, Nancharaiah YV, Cennamo P, Esposito G, van Hullebusch ED, Lens PNL (2018) Comparative performance of anaerobic attached biofilm and granular sludge reactors for the treatment of model mine drainage wastewater containing selenate, sulfate and nickel. Chem Eng J 345:545–555
Wang X, Zhang G, Lan HC, Liu RP, Liu HJ, Qu JH (2017) Preparation of hollow Fe-Al binary metal oxyhydroxide for efficient aqueous fluoride removal. Colloid Surface A 520:580–589
Wang WB, Zhang HX, Shen JF, Ye MX (2018) Facile preparation of magnetic chitosan/poly (vinyl alcohol) hydrogel beads with excellent adsorption ability via freezing-thawing method. Colloid Surface A 553:672–680
Wang X, Xu H, Wang DS (2020) Mechanism of fluoride removal by AlCl3 and Al-13: the role of aluminum speciation. J Hazard Mater 398:122987
Wei XC, Viadero RC, Buzby KM (2005) Recovery of iron and aluminum from acid mine drainage by selective precipitation. Environ Eng Sci 22:745–755
Xiang W, Zhang GK, Zhang YL, Tang DD, Wang JT (2014) Synthesis and characterization of cotton-like Ca-Al-La composite as an adsorbent for fluoride removal. Chem Eng J 250:423–430
Yuan ZD, Zhang GQ, Ma X, Yu L, Wang X, Wang SF, Jia YF (2019) Rapid abiotic As removal from As-rich acid mine drainage: effect of pH, Fe/As molar ratio, oxygen, temperature, initial As concentration and neutralization reagent. Chem Eng J 378:122156
Zhang XQ, Guo Y, Li WC (2015) Efficient removal of hexavalent chromium by high surface area Al2O3 rods. Rsc Adv 5:25896–25903
Zhao B, Xu XY, Zeng FQ, Li HB, Chen X (2018) The hierarchical porous structure bio-char assessments produced by co-pyrolysis of municipal sewage sludge and hazelnut shell and Cu(II) adsorption kinetics. Environ Sci Pollut R 25:19423–19435
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by the Basic Research Project of the Korea Institute of Geoscience and Mineral Resources (Project code: 21-3412-1).
Author information
Authors and Affiliations
Contributions
DWC: conceptualization, methodology, verification, writing—original draft preparation and editing; JYJ: investigation; SJ: validation; YWC: validation; GJY: supervision, resources, writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 543 kb)
Rights and permissions
About this article
Cite this article
Cho, W., Jang, JY., Ji, S. et al. Fabrication of aluminum beads derived from selectively recovered Al-rich precipitates and their application into defluoridation. Environ Sci Pollut Res 29, 999–1008 (2022). https://doi.org/10.1007/s11356-021-15727-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15727-z