Abstract
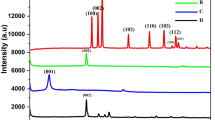
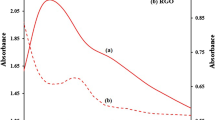
A new electrode was constructed via the anodic electropolymerization of poly-(L-serine) (PLS) on an rGO−Nafion–modified glassy carbon electrode (GCE) for the detection of the emerging organic contaminant naproxen (NPX). The morphology, crystal phase, and surface elements of the electrode were investigated with SEM, TEM, XRD, Raman, ATR-FTIR, zeta potential, C−H−O, and XPS analyses. Results of the surface analysis showed a porous structure resembling graphene sheets inside the Nafion/GCE architecture. Various electrochemical parameters, including scan rate, pH, and NPX concentration, were studied to evaluate the performance of the electrode. The synergistic effect of PLS and rGO−Nafion greatly facilitated the catalytic oxidation of NPX on PLS/rGO−Nafion/GCE. Electrochemical NPX oxidation was a one-electron transfer and adsorption limited process. The optimal working potential was 0.92 V vs. Ag/AgCl. The oxidation current of NPX increased with the increase in the concentration of analyte and scan rate but decreased with pH. The modified electrode exhibited excellent linearity with respect to NPX concentration in the range of 4.3 to 87 μM and limit of detection of 0.23 μM (S/N = 3). The PLS/rGO−Nafion/GCE is a fast, sensitive, reliable, and economical electrode for the detection of NPX in water.







Similar content being viewed by others
References
Adhoum N, Monser L, Toumi M, Boujlel K (2003) Determination of naproxen in pharmaceuticals by differential pulse voltammetry at a platinum electrode. Anal Chim Acta 495:69–75
Afzali F, Rounaghi G, Zavar M, Ashraf N (2016) Supramolecular β-cyclodextrin/multi-walled carbon nanotube paste electrode for amperometric detection of naproxen. J Electrochem Soc 163:B56–B61
Afzali M, Jahromi Z, Nekooie R (2019) Sensitive voltammetric method for the determination of naproxen at the surface of carbon nanofiber/gold/polyaniline nanocomposite modified carbon ionic liquid electrode. Microchem J 145:373–379
Aguilar-Lira GY, Álvarez Romero GA, Rojas-Hernández A, Páez-Hernández ME, Rodríguez-Ávila JA, Romero-Romo MA (2014) Voltammetric analysis of naproxen in graphite electrodes and its determination in pharmaceutical samples. Electroanalysis 32:1573–1581
Aguilar-Lira GY, Rojas-Hernández A, Rodríguez-Ávila JA, Páez-Hernández ME, Álvarez Romero GA (2020) Optimized quantification of naproxen based on DPV and a multiwalled MWCNT-carbon paste electrode. J Electrochem Soc 167:166510
Alavi-Tabaria SAR, Khalilzadeh MA, Karimi-Maleh H (2018) Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J Electroanal Chem 811:84–88
Aok KJ, Chen J (2018) Tips of voltammetry. Voltammetry. https://doi.org/10.5772/intechopen.81341
Asadian E, Ghalkhani M, Shahrokhian S (2019) Electrochemical sensing based on carbon nanoparticles: a review. Sensors Actuators B Chem 293:183–209
Bosca F, Martínez-Mánez R, Miranda MA, Primo J, Soto J, Vaño L (2012) Oxidative decarboxylation of naproxen. J Pharm Sci 81:479–482
Bui TAN, Nguyen TG, Darmanto W, Doong RA (2020) 3-Dimensional ordered reduced graphene oxide embedded with N-doped graphene quantum dots for high performance. Electrochim Acta 361:137018
Costentin C, Robert M, Savéant J (2010) Update 1 of: Electrochemical approach to the mechanistic study of proton-coupled electron transfer. Chem Rev 110:1–40
Do Prado TM, Badaró CC, Machado RG, Fadini PS, Fatibello-Filho O, Moraes FC (2020) Using bismuth vanadate/copper oxide nanocomposite as photoelectrochemical sensor for naproxen determination in sewage. Electroanalysis 32:1930–1937
Dong CD, Chen CW, Chen CF, Hung CM (2014a) Synthesis of platinum particles supported on microporous carbons for electrocatalytic of ammonia and its cytotoxicity study. J Adv Oxid Technol 17:17–24
Dong CD, Chen CW, Chen CF, Lai WL, Hung CM (2014b) Preparation and electrocatalytic properties of copper-based rare earth nanocomposites for use in methanol electrooxidation. J. Rare Earth 32:21–28
Dong CD, Chen CW, Chen CF, Hung CM (2014c) Platinum particles supported on mesoporous carbons: fabrication and electrocatalytic performance in methanol-tolerant oxygen-reduction reactions. Sci Rep 4:5790
Dong CD, Chen CW, Chen CF, Hung CM (2014d) Synthesis of platinum particles supported on microporous carbons for electrocatalytic of ammonia and its cytotoxicity study. J Adv Oxid Technol 17:17–24
Dong CD, Chen CW, Chen CF, Lai WL, Hung CM (2015) Material characterization and electrochemical performance of copper-based rare earth composite oxide electrodes for use in ammonia electrocatalytic oxidation. Desalination Water Treat 54:1054–1060
Dong CD, Chen CW, Hung CM (2016) Preparation, physicochemical and electrochemical properties of magnetite electrodes for methanol electrocatalytic oxidation in an alkaline medium. Desalination Water Treat 5:29404–29410
Dong CD, Chen CW, Kao CM, Hung CM (2017a) Synthesis, characterization, and application of CuO-modified TiO2 electrode exemplified for ammonia electro-oxidation. Process Saf Environ Prot 112:243–253
Dong CD, Chen CW, Hung CM (2017b) Cu-ACF composite catalyst: synthesis, characterization, and electrocatalytic properties towards ammonia oxidation in acid solution. J Hazard Toxic Radioact Waste 21:1–7
Elsie S, Green A, Rubavathi D, Angamuthu A, Gopal B, Bhagavathsingh J (2019) Tris-(2-aminoethyl)amine-intercalated graphene oxide as an efficient 2D material for cerium-ion fluorescent sensor applications. ACS Omega 4:22431–22437
Erdoğdu G (2007) Voltammetric study of the hydrolysis product of fenthion at the Nafion®-modified glassy carbon electrode. J Anal Chem 62:466–469
Fayyaz A, Saravanakumar K, Talukdar K, Kim Y, Yoon Y, Park CM (2021) Catalytic oxidation of naproxen in cobalt spinel ferrite decorated Ti3C2Tx MXene activated persulfate system: mechanisms and pathways. Chem Eng J 407:127842
Fonseca WT, Santos RF, Alves JN, Ribeiro SD, Takeuchi RM, Santos AL, Assunção RMN, Filho GR, Muñoz AA (2015) Square-wave voltammetry as analytical tool for real-time study of controlled naproxen releasing from cellulose derivative materials. Electroanalysis 27:1847–1854
Ganguly A, Sharma S, Papakonstantinou P, Hamilton J (2011) Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J Phys Chem C 115:17009–17019
García MG, Fernández-López C, Polesel F, Trapp S (2019) Predicting the uptake of emerging organic contaminants in vegetables irrigated with treated wastewater — implications for food safety assessment. Environ Res 172:175–181
Halling-Sørensen B, Nielsen SN, Lanzky PF, Ingerslev F, Lützhøft HCH, Jørgensen SE (1998) Occurrence, fate and effects of pharmaceutical substances in the environment—a review. Chemosphere 36:357–393
Hung CM (2012a) Complex PtPdRh nanoparticles: synthesis, characterization, and performance in electrocatalytic oxidation processes of ammonia. Powder Technol 232:18–23
Hung CM (2012b) Electrochemical properties of PtPdRh alloy catalysts for ammonia electrocatalytic oxidation. Int J Hydrog Energy 37:13815–13821
Hung CM (2012c) The study of catalytic oxidation ammonia reactivity using bimetallic PtRh particles as catalyst: electrocatalytic and electrochemical behavior. Adv Sci Lett 8:578–582
Hung CM (2013) Development of copper-lanthanum oxide catalyst for gaseous ammonia removal by catalytic oxidation: physicochemical and electrochemical characterization of catalyst materials. Int J Energy Res 37:2001–2008
Hung CM, Lai WL, Lin JL (2013) Investigation of fluorescence characterization and electrochemical behavior on the catalysts of nanosized Pt-Rh/γ-Al2O3 to oxidize gaseous ammonia. Front Environ Sci Eng 7:428–434
Hung CM, Huang CP, Chen SK, Chen CW, Dong CD (2020) Electrochemical analysis of naproxen in water using poly(L-serine)-modified glassy carbon electrode. Chemosphere 254:126686
Jahani PM, Javar HA, Mahmoudi-Moghaddam H (2020) Development of a novel electrochemical sensor using the FeNi3/CuS/BiOCl nanocomposite for determination of naproxen. J Mater Sci Mater Electron 31:14022–14034
Jung JH, Jeon JH, Sridhar V, Oh IK (2011) Electro-active graphene–Nafion actuators. Carbon 49:1279–1289
Kanagasabapathy M, Sekar R (2019) Chronopotentiometric/chronoamperometric transient analysis of naproxen via electrochemically synthesized nano spinel ZnFe2O4 films. J Electroanal Chem 832:59–68
Karimi-Maleh H, Karimi F, Orooji Y, Mansouri G, Razmjou A, Aygun A, Sen F (2020) A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid: a highly sensitive approach for determination of cysteamine in the presence of serotonin. Sci Rep 10:11699
Karimi-Maleh H, Orooji Y, Karimi F, Alizadeh M, Baghayeri M, Rouhi J, Tajik S, Beitollahi H, Agarwal S, Gupta VK, Rajendran S, Ayati A, Fu L, Sanati AL, Tanhaei B, Sen F, Shabani-nooshabadip M, Asrami PN, Al-Othman A (2021a) A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens Bioelectron 184:113252
Karimi-Maleh H, Yola ML, Atar N, Orooji Y, Karimi F, Kumar PS, Rouhi J, Baghayeri M (2021b) A novel detection method for organophosphorus insecticide fenamiphos: Molecularly imprinted electrochemical sensor based on core-shell Co3O4@MOF-74 nanocomposite. J Colloid Interface Sci 592:174–185
Karimi-Maleh H, Alizadeh M, Orooji Y, Karimi F, Baghayeri M, Rouhi J, Tajik S, Beitollahi H, Agarwal S, Gupta VK, Rajendran S, Rostamnia S, Fu L, Saberi-Movahed F, Malekmohammadi S (2021c) Guanine-based DNA biosensor amplified with Pt/SWCNTs nanocomposite as analytical tool for nanomolar determination of daunorubicin as an anticancer drug: a docking/experimental investigation. Ind Eng Chem Res 60:816–823
Kim D, Lee S, Piao Y (2017) Electrochemical determination of dopamine and acetaminophen using activated graphene-Nafion modified glassy carbon electrode. J Electroanal Chem 794:221–228
Li C (2006) Voltammetric determination of tyrosine based on an l-serine polymer film electrode. Colloid Surf. B: Biointerfaces 50:147–151
Liu Y, Liu Y, Liu Z, Zhao X, Wei J, Liu H, Si X, Xu Z, Cai Z (2020) Chiral molecularly imprinted polymeric stir bar sorptive extraction for naproxen enantiomer detection in PPCPs. J Hazard Mater 392:122251
López-Cázares MI, Isaacs-Páez ED, Ascacio-Valdés J, Aguilar-González CN, Rangel-Mendez JR, Chazaro-Ruiz LF (2021) Electro-assisted naproxen adsorption followed by its electrodegradation and simultaneous electroreactivation of the activated carbon electrode. Sep Purif Technol 258:118030
Montes RHO, Stefano JS, Richter EM, Munoz RAA (2014) Exploring multiwalled carbon nanotubes for naproxen detection. Electroanalysis 32:1449–1453
Norouzi P, Dousty F, Ganjali MR, Daneshgar P (2009) Dysprosium nanowire modified carbon paste electrode for the simultaneous determination of naproxen and paracetamol: application in pharmaceutical formulation and biological fluid. Int J Electrochem Sci 4:1373–1386
Pai CW, Leong D, Chen CY, Wang GS (2020) Occurrences of pharmaceuticals and personal care products in the drinking water of Taiwan and their removal in conventional water treatment processes. Chemosphere 256:127002
Pap S, Taggart MA, Shearer L, Li Y, Radovic S, Sekulic MT (2021) Removal behaviour of NSAIDs from wastewater using a P-functionalised microporous carbon. Chemosphere 264:128439
Qian L, Thiruppathi AR, Elmahdy R, van der Zalm J, Chen A (2020) Graphene-oxide-based electrochemical sensors for the sensitive detection of pharmaceutical drug naproxen. Sensors 20:1252
Ramírez-Morales D, Masís-Mora M, Beita-Sandí W, Montiel-Mora JR, Fernández-Fernández E, Méndez-Rivera M, Arias-Mora V, Leiva-Salas A, Brenes-Alfaro L, Rodríguez-Rodríguez CE (2021) Pharmaceuticals in farms and surrounding surface water bodies: hazard and ecotoxicity in a swine production area in Costa Rica. Chemosphere 2726:129574
Shao Y, Zhang S, Engelhard MH, Li G, Shao G, Wang Y, Liu J, Aksayc IA, Lin Y (2010) Nitrogen-doped graphene and its electrochemical applications. J Mater Chem 20:7491–7496
Soltani N, Tavakkoli N, Mosavimanesh ZS, Davar F (2018) Electrochemical determination of naproxen in the presence of acetaminophen using a carbon paste electrode modified with activated carbon nanoparticles. C R Chimie 21:54–60
Song J, Yang J, Hu X (2008) Electrochemical determination of estradiol using a poly (L-serine) film-modified electrode. J Appl Electrochem 38:833–836
Suryanarayanan V, Zhang Y, Yoshihara S, Shirakashi T (2005) Voltammetric assay of naproxen in pharmaceutical formulations using boron-doped diamond electrode. Electroanalysis 17:925–932
Tahir S, Yasmeen K, Hanif M, Khaliq O, Muhammad H, Hafsa IAT, Jahangir S, Ali ST (2019) Electrochemical methodology for NSAID’s determination and its interaction with steroid dexamethasone. Int J Electrochem Sci 14:5748–5762
Tarahomi S, Rounaghi GH, Daneshvar L (2019) A novel disposable sensor based on gold digital versatile disc chip modified with graphene oxide decorated with Ag nanoparticles/β-cyclodextrin for voltammetric measurement of naproxen. Sensors Actuators B Chem 286:445–450
Tashkhourian J, Hemmateenejad B, Beigizadeh H, Hosseini-Sarvari M, Razmi Z (2014) ZnO nanoparticles and multiwalled carbon nanotubes modified carbon paste electrode for determination of naproxen using electrochemical techniques. J Electroanal Chem 714-715:103–108
Ternes TA (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res 32:3254–3260
Tixier C, Singer HP, Oellers S, Müller SR (2003) Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ Sci Technol 37:1061–1068
Tu N, Liu Y, Li R, Lv W, Liu G, Ma D (2019) Experimental and theoretical investigation on photodegradation mechanisms of naproxen and its photoproducts. Chemosphere 227:142–150
Walden AG, Miler AJM (2015) Rapid water oxidation electrocatalysis by a ruthenium complex of the tripodal ligand tris(2-pyridyl)phosphine oxide. Chem Sci 6:2405–2410
Wang J, 2000 Analytical electrochemistry, Willey-VCH, 2nd Edition
Wang CY, Yu B, Fu H, Wang P, Wang CC (2019) A mixed valence Tb(III)/Tb(IV) metal–organic framework: crystal structure, luminescence property and selective detection of naproxen. Polyhedron 159:298–307
Wojcieszyńska D, Guzik U (2020) Naproxen in the environment: its occurrence, toxicity to nontarget organisms and biodegradation. Appl Microbiol Biotechnol 104:1849–1857
Wu D, Sui Q, Yu X, Zhao W, Li Q, Fatta-Kassinos D, Lyu S (2021) Identification of indicator PPCPs in landfill leachates and livestock wastewaters usingmulti-residue analysis of 70 PPCPs: analytical method development and application in Yangtze River Delta. China Sci Total Environ 753:141653
Yang S, Wang G, Li G, Qu L (2015) One step controllable electrochemical deposition of silver hexacyanoferrate nanoparticles/multi-wall carbon nanotubes/Nafion modified electrode for the sensing of phenol. J Anal Chem 70:1116–1122
Yiğit A, Yardım Y, Çelebi M, Levent A, Şentürk Z (2016) Graphene/Nafion composite film modified glassy carbon electrode for simultaneous determination of paracetamol, aspirin and caffeine in pharmaceutical formulations. Talanta 158:21–29
Zarezadeh A, Rajabi HR, Sheydaei O, Khajehsharifi H (2019) Application of a nano-structured molecularly imprinted polymer as an efficient modifier for the design of captopril drug selective sensor: mechanism study and quantitative determination. Mater Sci Eng C 94:879–885
Acknowledgements
The authors would like to thank Shih-Kai Chen of the National Kaohsiung University of Science and Technology for assistance with voltammetry measurements.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
Conceptualization: CDD, CPH, CWC, and CMH. Background and data collection: CMH. Validation: CMH, CPH, and CDD. Methodology: CDD and CWC. Formal analysis: CMH. Data curation: CMH, CPH, CWC, and CDD. Writing—original draft preparation: CMH. Writing review and editing: CDD and CPH. Visualization: CMH, CPH, CWC, and CDD. Supervision: CWC and CDD. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 158 kb)
Rights and permissions
About this article
Cite this article
Hung, M., Huang, CP., Chen, CW. et al. A poly-(L-serine)/reduced graphene oxide–Nafion supported on glassy carbon (PLS/rGO−Nafion/GCE) electrode for the detection of naproxen in aqueous solutions. Environ Sci Pollut Res 29, 12450–12461 (2022). https://doi.org/10.1007/s11356-021-15511-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15511-z