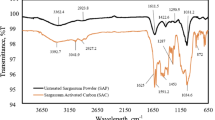
Abstract
In this research work, the biosorption potential of brown algae, Sargassum polycystum, was investigated for the removal of toxic metals, cadmium (Cd) and zinc (Zn), under controlled environmental conditions. The biosorbent prepared from the S. polycystum was characterized by Brunauer-Emmett-Teller (BET), scanning electron microscope (SEM) and Fourier transform infrared spectroscopy (FTIR) techniques. The optimal conditions identified using Box-Behnken design (BBD) for Cd removal were pH: 4.65, biosorbent mass: 1.8 g/L and shaking speed: 76 rpm. For zinc, the optimum values were pH: 5.7, biosorbent mass: 1.2 g/L and shaking speed: 125 rpm, respectively. The equilibrium uptake of the metals, Cd and Zn, was evaluated by isotherm models. The Langmuir isotherm proved to be an excellent fit confirming single layer of sorption. The maximum Cd and Zn uptakes achieved were 105.26 mg/g and 116.2 mg/g respectively. The kinetics of Cd and Zn biosorption onto brown algae Sargassum polycystum, follows pseudo-second order. The thermodynamic parameters were determined, and the sorption process was found to be feasible. Desorption studies of Cd and Zn were performed, and the bio sorbent reproduced appreciable efficiency for five successive cycles of sorption-desorption process using HCl.









Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BD :
-
D-R constant
- bT :
-
Temkin constant
- C:
-
Intercept
- Ce :
-
Equilibrium concentration of the metal in the solution (mg/L)
- C0 :
-
Initial metal ion concentration (mg/L)
- K:
-
Equilibrium constant
- KF :
-
Freundlich isotherm constant
- kL :
-
Langmuir sorption constant (L/mg)
- KT :
-
Equilibrium binding constant in L/mg
- k1 :
-
Rate constant of the first-order equation (min−1)
- k2 :
-
Rate constant of second-order equation(g/mg min)
- Kid :
-
Intra particle diffusion rate constant in mg g−1 min−0.5
- qe, qm qt :
-
Amount of the metal ions sorbed at equilibrium, maximum and at given time (mg/g)
- qmax :
-
Monolayer sorption capacity (mg/g)
- R:
-
Universal gas constant 8.314 × 10−3 in kJ/mol K
- T:
-
Temperature in Kelvin
- T:
-
Time (min)
- V:
-
Volume of solution (L)
- W:
-
Mass of biosorbent (g)
- 1/n:
-
Heterogeneity factor
- ∆G ͦ :
-
Gibbs free energy (kJ/mol)
- ∆H ͦ :
-
Enthalpy change (kJ/mol)
- ∆S ͦ :
-
Entropy change (kJ/mol K)
References
Ayabei K, Kituyi L (2013) Comparative study of rates of biosorption for selected single and mixed metal ions using natural products. Chem Mater Res 3:1–7
Baghdad K, Hasnaoui AM (2020) Zeolite–cellulose composite membranes: synthesis and applications in metals and bacteria removal. J Environ Chem Eng 8:104047. https://doi.org/10.1016/j.jece.2020.104047
Bora AJ, Dutta RK (2019) Removal of metals (Pb, Cd, Cu, Cr, Ni, and Co) from drinking water by oxidation-coagulation-absorption at optimized pH. J Water Process Eng 31:100839. https://doi.org/10.1016/j.jwpe.2019.100839
Centeno SA, Shamir J (2008) Surface enhanced Raman scattering (SERS) and FTIR characterization of the sepia melanin pigment used in works of art. J Mol Struct 873:149–159. https://doi.org/10.1016/j.molstruc.2007.03.026
Chegrouche S, Mellah A, Barkat M (2009) Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalination 235:306–318. https://doi.org/10.1016/j.desal.2008.01.018
Chen Q, Yao Y, Li X, Lu J, Zhou J, Huang Z (2018) Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J Water Process Eng 26:289–300. https://doi.org/10.1016/j.jwpe.2018.11.003
Cortes LN, Druzian SP, Streit AFM, Cadaval TRS Jr, Collazzo GC, Dotto GL (2019) Preparation of carbonaceous materials from pyrolysis of chicken bones and its application for fuchsine adsorption. Environ Sci Pollut Res 26:28574–28583. https://doi.org/10.1007/s11356-018-3679-2
Deng L, Su Y, Su H, Wang X, Zhu X (2007) Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J Hazard Mater 143:220–225. https://doi.org/10.1016/j.jhazmat.2006.09.009
Freitas MMO, Martins RJE, Delerue-Matos CM, Boaventura RAR (2008) Removal of Cd (II), Zn(II) and Pb(II) from aqueous solutions by brown marine macro algae: kinetic modeling. J Hazard Mater 153:493–501. https://doi.org/10.1016/j.jhazmat.2007.08.081
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Giles CH, Macewan TH, Nakhwa SN, Smith D (1960) Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Chem Soc 786:3973–3993. https://doi.org/10.1039/JR9600003973
Goyal P, Tiwary CS, Misra SK (2021) Ion exchange based approach for rapid and selective Pb(II) removal using iron oxide decorated metal organic framework hybrid. J Environ Manage 277:111469. https://doi.org/10.1016/j.jenvman.2020.111469
Hashim MA, Chu KH (2004) Biosorption of cadmium by brown, green, and red seaweeds. Chem Eng J 97:249–255. https://doi.org/10.1016/S1385-8947(03)00216-X
He J, Chen P (2014) A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Bioresour Technol 160:67–78. https://doi.org/10.1016/j.biortech.2014.01.068
Jayakumar R, Rajasimman M, Karthikeyan C (2014) Sorption of hexavalent chromium from aqueous solution using marine green algae Halimeda gracilis: optimization, equilibrium, kinetic, thermodynamic and desorption studies. J Environ Chem Eng 2:1261–1274. https://doi.org/10.1016/j.jece.2014.05.007
Jayakumar R, Rajasimman M, Karthikeyan C (2015) Sorption and desorption of hexavalent chromium using a novel brown marine algae Sargassum myriocystum. Korean J Chem Eng 32:2031–2046. https://doi.org/10.1007/s11814-015-0036-8
Jayakumar V, Govindaradjane S, Rajasimman M (2019) Isotherm and kinetic modeling of sorption of Cadmium onto a novel red algal sorbent Hypnea musciformis. Model Earth Syst Environ 5:793–805. https://doi.org/10.1007/s40808-018-0566-2
Jayakumar V, Govindaradjane S, Rajasimman M (2020) Efficient adsorptive removal of zinc by green marine macro alga Caulerpa scalpelliformis—characterization, optimization, modeling, isotherm, kinetic, thermodynamic, desorption and regeneration studies. Surf Interfaces 22:100798. https://doi.org/10.1016/j.surfin.2020.100798
Jin-fen P, Rong-gen L, Li M (2000) A review of heavy metal adsorption by marine algae. Chin J Ocean Limnol 18:260–264. https://doi.org/10.1007/BF02842673
Joo JH, Hassan SH, Oh SE (2010) Comparative study of biosorption of Zn2+ by Pseudomonas aeruginosa and Bacillus cereus. Int Biodeter Biodegr 64:734–741. https://doi.org/10.1016/j.ibiod.2010.08.007
Langmuir I (1916) The adsorption of gases on plane surface of glass, mica and platinum. J American Chem Soc 40:1361–1368
Lodeiro P, Cordero B, Barriada JL, Herrero R, Sastre de Vicente ME (2005) Biosorption of cadmium by biomass of brown marine macroalgae. Bioresour Technol 96:1796–1803. https://doi.org/10.1016/j.biortech.2005.01.002
Luo F, Liu Y, Cao Q, Chen J (2009) Biosorption of Cd2+, Cu2+, Ni2+ and Zn2+ ions from aqueous solutions by pretreated biomass of brown algae. J Hazard Mater 163:931–938. https://doi.org/10.1016/j.jhazmat.2008.07.046
Martin JA, Solla A, Coimbra MA, Gil L (2005) Metabolic distinction of Ulmus minor xylem tissues after inoculation with Ophiostoma novo-ulmi. Phytochemistry 66:2458–2467. https://doi.org/10.1016/j.phytochem.2005.08.004
Mehta SK, Gaur JP (2005) Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit Rev Biotechnol 25:113–152. https://doi.org/10.1080/07388550500248571
Michalak I, Chojnacka K, Witek-Krowiak A (2013) State of the art for the biosorption process—a review. Appl Biochem Biotechnol 170:1389–1416. https://doi.org/10.1007/s12010-013-0269-0
Montazer MM, Rahmati PR, Abdolali A, Keshtkar AR (2011) Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. J Hazard Mater 185:401–407. https://doi.org/10.1016/j.jhazmat.2010.09.047
Muller U, Diem M (1993) Introduction to modern vibrational spectroscopy. J Wiley, New York, Chichester, 1993, ISBN 0-471-59584-5, 285 Seiten
Nuhoglu Y, Oguz E (2003) Removal of copper (II) from aqueous solutions by biosorption on the cone biomass of Thuja orientalis. Process Biochem 38:1627–1631. https://doi.org/10.1016/S0032-9592(03)00055-4
Nystrom F, Nordqvist K, Herrmann I, Hedstrom A, MariaViklander (2020) Removal of metals and hydrocarbons from stormwater using coagulation and flocculation. Water Res 182:115919. https://doi.org/10.1016/j.watres.2020.115919
Park D, Yun YS, Park JM (2005) Use of dead fungal biomass for the detoxification of hexavalent chromium: screen and kinetics. Process Biochem 40:2559–2565. https://doi.org/10.1016/j.procbio.2004.12.002
Rajamohan N, Rajasimman M (2015) Biosorption of Selenium using activated plant based sorbent—effect of variables, isotherm and kinetic modeling. Biocatal Agric Biotechnol 4:795–800. https://doi.org/10.1016/j.bcab.2015.10.013
Rajamohan N, Rajasimman M, Dilipkumar M (2014a) Parametric and kinetic studies on biosorption of mercury using modified Phoenix dactylifera biomass. J Taiwan Inst Chem Eng 45:2622–2627. https://doi.org/10.1016/j.jtice.2014.07.004
Rajamohan N, Rajasimman M, Rajeshkannan R, Saravanan V (2014b) Equilibrium, kinetic and thermodynamic studies on the removal of aluminum by modified Eucalyptus camaldulensis barks. Alex Eng J 53:409–415. https://doi.org/10.1016/j.aej.2014.01.007
Rajasimman M, Murugaiyan K (2010) Optimization of process variables for the biosorption of chromium using Hypnea Valentiae. Nova Biotechnologica 10:107–115
Rajasimman M, Sangeetha R, Karthic P (2009) Statistical optimization of process parameters for the extraction of chromium (VI) from pharmaceutical wastewater by emulsion liquid membrane. Chem Eng J 150:275–279. https://doi.org/10.1016/j.cej.2008.12.026
Redha AA (2020) Removal of heavy metals from aqueous media by biosorption. Arab J Basic Appl Sci 27:183–193. https://doi.org/10.1080/25765299.2020.1756177
Rincon GJ, Motta EJL (2014) Simultaneous removal of oil and grease, and heavy metals from artificial bilge water using electro-coagulation/flotation. J Environ Manage 144:42–50. https://doi.org/10.1016/j.jenvman.2014.05.004
Romera E, Gonzalez F, Ballester A, Blazquez ML, Munoz JA (2007) Comparative study of biosorption of heavy metals using different types of algae. Bioresour Technol 98:3344–3353. https://doi.org/10.1016/j.biortech.2006.09.026
Rosa MA, Egido JA, Marquez MC (2017) Enhanced electrochemical removal of arsenic and heavy metals from mine tailings. J Taiwan Inst Chem Eng 78:409–415. https://doi.org/10.1016/j.jtice.2017.06.046
Sheng PX, Ting Y-P, Paul Chen J, Liang H (2004) Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci 275:131–141. https://doi.org/10.1016/j.jcis.2004.01.036
Su H, Li J, Tan T (2008) Adsorption mechanism for imprinted ion (Ni2+) of the surface molecular imprinting adsorbent (SMIA). Biochem Eng J 39:503–509. https://doi.org/10.1016/j.bej.2007.11.011
Sun Y, Zhou S, Sun W, Zhu S, Zheng H (2020) Flocculation activity and evaluation of chitosan-based flocculant CMCTS-g-P(AM-CA) for heavy metal removal. Sep Purif Technol 241:116737. https://doi.org/10.1016/j.seppur.2020.116737
Temkin MJ, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiol Chem 12:217–256
US Environmental Protection Agency (1999) Integrated Risk Information System (IRIS) on Cadmium, National Centre for Environmental Assessment. Office of Research and Development, Washington, DC
Verma A, Kumar S, Kumar S (2017) Statistical modeling, equilibrium and kinetic studies of cadmium ions biosorption from aqueous solution using S. filipendula. J Environ Chem Eng 5:2290–2304. https://doi.org/10.1016/j.jece.2017.03.044
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution, Journal of the Sanitary Engineering Division. Am Soc Civil Eng 89:31–60
Yavuz H, Denizli A, Gungunes H, Safarikova M, Safarik I (2006) Biosorption of mercury on magnetically modified yeast cells. Sep Purif Technol 52:253–260. https://doi.org/10.1016/j.seppur.2006.05.001
Author information
Authors and Affiliations
Contributions
V.J.—conceptualization, data curation, formal analysis, investigation, validation, writing—original draft; S.G.—resources, supervision; N.R.—resources, writing—review & editing; M.R.—methodology, software, visualization, writing—review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jayakumar, V., Govindaradjane, S., Rajamohan, N. et al. Biosorption potential of brown algae, Sargassum polycystum, for the removal of toxic metals, cadmium and zinc. Environ Sci Pollut Res 29, 41909–41922 (2022). https://doi.org/10.1007/s11356-021-15185-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15185-7